The tick-over theory revisited: formation and regulation of the soluble alternative complement C3 convertase (C3(H2O)Bb)
- PMID: 18096230
- PMCID: PMC2701500
- DOI: 10.1016/j.molimm.2007.11.003
The tick-over theory revisited: formation and regulation of the soluble alternative complement C3 convertase (C3(H2O)Bb)
Abstract
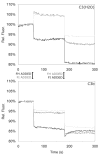
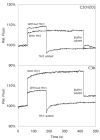
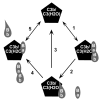
The molecular interactions between the components of the C3 convertase of the alternative pathway (AP) of complement and its regulators, in both surface-bound and fluid-phase form, are still incompletely understood. The fact that the AP convertase is labile makes studies difficult to perform. According to the so called tick-over theory, hydrolyzed C3, called C3(H(2)O), forms the initial convertase in fluid phase together with factor B. In the present study, we have applied western blot analysis and ELISA together with fluorescence resonance energy transfer (FRET) to study the formation of the fluid-phase AP convertases C3(H(2)O)Bb and C3bBb and their regulation by factor H and factor I at specific time points and, with FRET, in real time. In our hands, factor B showed a higher affinity for C3(H(2)O) than for C3b, although in both cases it was readily activated to Bb. However, the convertase activity of C3bBb was approximately twice that of C3(H(2)O)Bb, as monitored by the generation of C3a. But in contrast, the C3(H(2)O)Bb convertase was more resistant to inactivation by factor H and factor I than was the C3bBb convertase. Under conditions that totally inactivated C3bBb, C3(H(2)O)Bb still retained approximately 25% of its initial activity.
Figures




References
-
- Andersson J, Ekdahl KN, Lambris JD, Nilsson B. Binding of C3 fragments on top of adsorbed plasma proteins during complement activation on a model biomaterial surface. Biomaterials. 2005;26:1477–85. - PubMed
-
- Andersson J, Ekdahl KN, Larsson R, Nilsson UR, Nilsson B. C3 adsorbed to a polymer surface can form an initiating alternative pathway convertase. J Immunol. 2002;168:5786–91. - PubMed
-
- Asghar SS, Barendsen CY, van der Helm HJ. Reinvestigations into the formation and assay of C3bBbP complexes. Clin Chim Acta. 1987;165:243–52. - PubMed
-
- Catana E, Schifferli JA. Purification of human complement factor D from the peritoneal fluid of patients on chronic ambulatory peritoneal dialysis. J Immunol Methods. 1991;138:265–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

