A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol
- PMID: 18096503
- PMCID: PMC2692834
- DOI: 10.1016/j.chembiol.2007.11.006
A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol
Abstract
Endogenous ligands for cannabinoid receptors ("endocannabinoids") include the lipid transmitters anandamide and 2-arachidonoylglycerol (2-AG). Endocannabinoids modulate a diverse set of physiological processes and are tightly regulated by enzymatic biosynthesis and degradation. Termination of anandamide signaling by fatty acid amide hydrolase (FAAH) is well characterized, but less is known about the inactivation of 2-AG, which can be hydrolyzed by multiple enzymes in vitro, including FAAH and monoacylglycerol lipase (MAGL). Here, we have taken a functional proteomic approach to comprehensively map 2-AG hydrolases in the mouse brain. Our data reveal that approximately 85% of brain 2-AG hydrolase activity can be ascribed to MAGL, and that the remaining 15% is mostly catalyzed by two uncharacterized enzymes, ABHD6 and ABHD12. Interestingly, MAGL, ABHD6, and ABHD12 display distinct subcellular distributions, suggesting that they may control different pools of 2-AG in the nervous system.
Figures

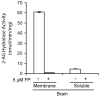



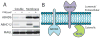
Comment in
-
2-AG + 2 new players = forecast for therapeutic advances.Chem Biol. 2007 Dec;14(12):1309-11. doi: 10.1016/j.chembiol.2007.12.004. Chem Biol. 2007. PMID: 18096497
References
-
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. - PubMed
-
- Di Marzo V, Bisogno T, De Petrocellis L. Endocannabinoids and Related Compounds: Walking Back and Forth between Plant Natural Products and Animal Physiology. Chemistry & Biology. 2007;14:741–756. - PubMed
-
- Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, Krey JF, Walker JM, Holmes PV, Crystal JD, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–1112. - PubMed
-
- Di Marzo V, Goparaju SK, Wang L, Liu J, Batkai S, Jarai Z, Fezza F, Miura GI, Palmiter RD, Sugiura T, Kunos G. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. - PubMed
-
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

