GTP up-regulated persistent Na+ current and enhanced nociceptor excitability require NaV1.9
- PMID: 18096591
- PMCID: PMC2268982
- DOI: 10.1113/jphysiol.2007.147942
GTP up-regulated persistent Na+ current and enhanced nociceptor excitability require NaV1.9
Abstract
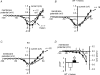
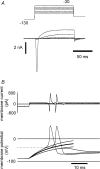
Persistent tetrodotoxin-resistant (TTX-r) sodium currents up-regulated by intracellular GTP have been invoked as the site of action of peripheral inflammatory mediators that lower pain thresholds, and ascribed to the Na(V)1.9 sodium channel. Here we describe the properties of a global knock-out of Na(V)1.9 produced by replacing exons 4 and 5 in SCN11A with a neomycin resistance cassette, deleting the domain 1 voltage sensor and introducing a frameshift mutation. Recordings from small (< 25 microm apparent diameter) sensory neurones indicated that channel loss eliminates a TTX-r persistent current. Intracellular dialysis of GTP-gamma-S did not cause an up-regulation of persistent Na(+) current in Na(V)1.9-null neurones and the concomitant negative shift in voltage-threshold seen in wild-type and heterozygous neurones. Heterologous hNa(V)1.9 expression in Na(V)1.9 knock-out sensory neurones confirms that the human clone can restore the persistent Na(+) current. Taken together, these findings demonstrate that Na(V)1.9 underlies the G-protein pathway-regulated TTX-r persistent Na(+) current in small diameter sensory neurones that may drive spontaneous discharge in nociceptive nerve fibres during inflammation.
Figures




Comment in
-
Nav1.9, G-proteins, and nociceptors.J Physiol. 2008 Feb 15;586(4):917-8. doi: 10.1113/jphysiol.2007.149922. J Physiol. 2008. PMID: 18287383 Free PMC article. No abstract available.
References
-
- Amaya F, Wang H, Costigan M, Allchorne AJ, Hatcher JP, Egerton J, Stean T, Morisset V, Grose D, Gunthorpe MJ, Chessell IP, Tate S, Green PJ, Woolf CJ. The voltage-gated sodium channel NaV1.9 is an effector of peripheral inflammatory pain hypersensitivity. J Neurosci. 2006;26:12852–12860. - PMC - PubMed
-
- Baker MD, Bostock H. Low-threshold persistent sodium current in rat large dorsal root ganglion neurones in culture. J Neurophysiol. 1997;77:1503–1513. - PubMed
-
- Black JA, Waxman SG. Molecular identities of two tetrodotoxin-resistant sodium channels in corneal axons. Exp Eye Res. 2002;75:193–199. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

