Regulation of synaptic signalling by postsynaptic, non-glutamate receptor ion channels
- PMID: 18096597
- PMCID: PMC2375695
- DOI: 10.1113/jphysiol.2007.148353
Regulation of synaptic signalling by postsynaptic, non-glutamate receptor ion channels
Abstract
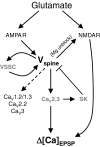
Activation of glutamatergic synapses onto pyramidal neurons produces a synaptic depolarization as well as a buildup of intracellular calcium (Ca(2+)). The synaptic depolarization propagates through the dendritic arbor and can be detected at the soma with a recording electrode. Current influx through AMPA-type glutamate receptors (AMPARs) provides the depolarizing drive, and the amplitudes of synaptic potentials are generally thought to reflect the number and properties of these receptors at each synapse. In contrast, synaptically evoked Ca(2+) transients are limited to the spine containing the active synapse and result primarily from Ca(2+) influx through NMDA-type glutamate receptors (NMDARs). Here we review recent studies that reveal that both synaptic depolarizations and spine head Ca(2+) transients are strongly regulated by the activity of postsynaptic, non-glutamate receptor ion channels. In hippocampal pyramidal neurons, voltage- and Ca(2+)-gated ion channels located in dendritic spines open as downstream consequences of glutamate receptor activation and act within a complex signalling loop that feeds back to regulate synaptic signals. Dynamic regulation of these ion channels offers a powerful mechanism of synaptic plasticity that is independent of direct modulation of glutamate receptors.
Figures


References
-
- Berkefeld H, Sailer CA, Bildl W, Rohde V, Thumfart JO, Eble S, Klugbauer N, Reisinger E, Bischofberger J, Oliver D, Knaus HG, Schulte U, Fakler B. BKCa-Cav channel complexes mediate rapid and localized Ca2+-activated K+ signaling. Science. 2006;314:615–620. - PubMed
-
- Bildl W, Strassmaier T, Thurm H, Andersen J, Eble S, Oliver D, Knipper M, Mann M, Schulte U, Adelman JP, Fakler B. Protein kinase CK2 is coassembled with small conductance Ca2+-activated K+ channels and regulates channel gating. Neuron. 2004;43:847–858. - PubMed
-
- Blake JF, Brown MW, Collingridge GL. CNQX blocks acidic amino acid induced depolarizations and synaptic components mediated by non-NMDA receptors in rat hippocampal slices. Neurosci Lett. 1988;89:182–186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

