Blunted response to low oxygen of rat respiratory network after perinatal ethanol exposure: involvement of inhibitory control
- PMID: 18096598
- PMCID: PMC2375658
- DOI: 10.1113/jphysiol.2007.147165
Blunted response to low oxygen of rat respiratory network after perinatal ethanol exposure: involvement of inhibitory control
Abstract
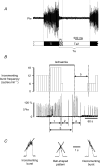
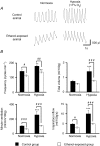
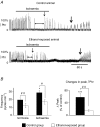
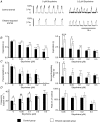
Acute ethanol depresses respiration, but little is known about chronic ethanol exposure during gestation and breathing, while the deleterious effects of ethanol on CNS development have been clearly described. In a recent study we demonstrated that pre- and postnatal ethanol exposure induced low minute ventilation in juvenile rats. The present study analysed in juvenile rats the respiratory response to hypoxia in vivo by plethysmography and the phrenic (Phr) nerve response to ischaemia in situ. Glycinergic neurotransmission was assessed in situ with strychnine application and [(3)H]strychnine binding experiments performed in the medulla. After chronic ethanol exposure, hyperventilation during hypoxia was blunted in vivo. In situ Phr nerve response to ischaemia was also impaired, while gasping activity occurred earlier and recovery was delayed. Strychnine applications in situ (0.05-0.5 microM) demonstrated a higher sensitivity of expiratory duration in ethanol-exposed animals compared to control animals. Moreover, [(3)H]strychnine binding density was increased after ethanol and was associated with higher affinity. Furthermore, 0.2 microM strychnine in ethanol-exposed animals restored the low basal Phr nerve frequency, but also the Phr nerve response to ischaemia and the time to recovery, while gasping activity appeared even earlier with a higher frequency. Polycythaemia was present after ethanol exposure whereas lung and heart weights were not altered. We conclude that chronic ethanol exposure during rat brain development (i) induced polycythaemia to compensate for low minute ventilation at rest; (ii) impaired the respiratory network adaptive response to low oxygen because of an increase in central glycinergic tonic inhibitions, and (iii) did not affect gasping mechanisms. We suggest that ethanol exposure during early life can be a risk factor for the newborn respiratory adaptive mechanisms to a low oxygen environment.
Figures
Comment in
-
Maternal drinking of alcohol: the newborn has the worst hangover.J Physiol. 2008 Mar 1;586(5):1201. doi: 10.1113/jphysiol.2008.150664. J Physiol. 2008. PMID: 18310127 Free PMC article. No abstract available.
References
-
- Aguayo LG, Pancetti FC. Ethanol modulation of the gamma-aminobutyric acidA- and glycine-activated Cl− current in cultured mouse neurons. J Pharmacol Exp Ther. 1994;270:61–69. - PubMed
-
- Aguayo LG, Tapia JC, Pancetti FC. Potentiation of the glycine-activated Cl− current by ethanol in cultured mouse spinal neurons. J Pharmacol Exp Ther. 1996;279:1116–1122. - PubMed
-
- Barker DJ. The developmental origins of adult disease. J Am Coll Nutr. 2004;6(Suppl.):588S–595S. - PubMed
-
- Bartlett D, Jr, Tenney SM. Control of breathing in experimental anemia. Respir Physiol. 1970;10:384–395. - PubMed
-
- Burd L, Klug M, Martsolf J. Increased sibling mortality in children with fetal alcohol syndrome. Addict Biol. 2004;9:179–188. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

