Unique fluorophores in the dimeric archaeal histones hMfB and hPyA1 reveal the impact of nonnative structure in a monomeric kinetic intermediate
- PMID: 18096639
- PMCID: PMC2222717
- DOI: 10.1110/ps.073224308
Unique fluorophores in the dimeric archaeal histones hMfB and hPyA1 reveal the impact of nonnative structure in a monomeric kinetic intermediate
Abstract
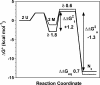
Homodimeric archaeal histones and heterodimeric eukaryotic histones share a conserved structure but fold through different kinetic mechanisms, with a correlation between faster folding/association rates and the population of kinetic intermediates. Wild-type hMfB (from Methanothermus fervidus) has no intrinsic fluorophores; Met35, which is Tyr in hyperthermophilic archaeal histones such as hPyA1 (from Pyrococcus strain GB-3A), was mutated to Tyr and Trp. Two Tyr-to-Trp mutants of hPyA1 were also characterized. All fluorophores were introduced into the long, central alpha-helix of the histone fold. Far-UV circular dichroism (CD) indicated that the fluorophores did not significantly alter the helical content of the histones. The equilibrium unfolding transitions of the histone variants were two-state, reversible processes, with DeltaG degrees (H2O) values within 1 kcal/mol of the wild-type dimers. The hPyA1 Trp variants fold by two-state kinetic mechanisms like wild-type hPyA1, but with increased folding and unfolding rates, suggesting that the mutated residues (Tyr-32 and Tyr-36) contribute to transition state structure. Like wild-type hMfB, M35Y and M35W hMfB fold by a three-state mechanism, with a stopped-flow CD burst-phase monomeric intermediate. The M35 mutants populate monomeric intermediates with increased secondary structure and stability but exhibit decreased folding rates; this suggests that nonnative interactions occur from burial of the hydrophobic Tyr and Trp residues in this kinetic intermediate. These results implicate the long central helix as a key component of the structure in the kinetic monomeric intermediates of hMfB as well as the dimerization transition state in the folding of hPyA1.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

