Killer artificial antigen-presenting cells: a novel strategy to delete specific T cells
- PMID: 18096763
- PMCID: PMC2275020
- DOI: 10.1182/blood-2007-09-113522
Killer artificial antigen-presenting cells: a novel strategy to delete specific T cells
Abstract
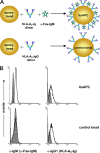
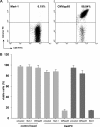
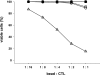
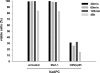
Several cell-based immunotherapy strategies have been developed to specifically modulate T cell-mediated immune responses. These methods frequently rely on the utilization of tolerogenic cell-based antigen-presenting cells (APCs). However, APCs are highly sensitive to cytotoxic T-cell responses, thus limiting their therapeutic capacity. Here, we describe a novel bead-based approach to modulate T-cell responses in an antigen-specific fashion. We have generated killer artificial APCs (kappaaAPCs) by coupling an apoptosis-inducing alpha-Fas (CD95) IgM mAb together with HLA-A2 Ig molecules onto beads. These kappaaAPCs deplete targeted antigen-specific T cells in a Fas/Fas ligand (FasL)-dependent fashion. T-cell depletion in cocultures is rapidly initiated (30 minutes), dependent on the amount of kappaaAPCs and independent of activation-induced cell death (AICD). kappaaAPCs represent a novel technology that can control T cell-mediated immune responses, and therefore has potential for use in treatment of autoimmune diseases and allograft rejection.
Figures

References
-
- Toungouz M, Donckier V, Goldman M. Tolerance induction in clinical transplantation: the pending questions. Transplantation. 2003;75:58S–60S. - PubMed
-
- Lechler RI, Sykes M, Thomson AW, Turka LA. Organ transplantation: how much of the promise has been realized? Nat Med. 2005;11:605–613. - PubMed
-
- Anderson G, Moore NC, Owen JJ, Jenkinson EJ. Cellular interactions in thymocyte development. Annu Rev Immunol. 1996;14:73–99. - PubMed
-
- Krammer PH. CD95's deadly mission in the immune system. Nature. 2000;407:789–795. - PubMed
-
- Askenasy N, Yolcu ES, Yaniv I, Shirwan H. Induction of tolerance using Fas ligand: a double-edged immunomodulator. Blood. 2005;105:1396–1404. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

