Isolation of rare circulating tumour cells in cancer patients by microchip technology
- PMID: 18097410
- PMCID: PMC3090667
- DOI: 10.1038/nature06385
Isolation of rare circulating tumour cells in cancer patients by microchip technology
Abstract
Viable tumour-derived epithelial cells (circulating tumour cells or CTCs) have been identified in peripheral blood from cancer patients and are probably the origin of intractable metastatic disease. Although extremely rare, CTCs represent a potential alternative to invasive biopsies as a source of tumour tissue for the detection, characterization and monitoring of non-haematologic cancers. The ability to identify, isolate, propagate and molecularly characterize CTC subpopulations could further the discovery of cancer stem cell biomarkers and expand the understanding of the biology of metastasis. Current strategies for isolating CTCs are limited to complex analytic approaches that generate very low yield and purity. Here we describe the development of a unique microfluidic platform (the 'CTC-chip') capable of efficient and selective separation of viable CTCs from peripheral whole blood samples, mediated by the interaction of target CTCs with antibody (EpCAM)-coated microposts under precisely controlled laminar flow conditions, and without requisite pre-labelling or processing of samples. The CTC-chip successfully identified CTCs in the peripheral blood of patients with metastatic lung, prostate, pancreatic, breast and colon cancer in 115 of 116 (99%) samples, with a range of 5-1,281 CTCs per ml and approximately 50% purity. In addition, CTCs were isolated in 7/7 patients with early-stage prostate cancer. Given the high sensitivity and specificity of the CTC-chip, we tested its potential utility in monitoring response to anti-cancer therapy. In a small cohort of patients with metastatic cancer undergoing systemic treatment, temporal changes in CTC numbers correlated reasonably well with the clinical course of disease as measured by standard radiographic methods. Thus, the CTC-chip provides a new and effective tool for accurate identification and measurement of CTCs in patients with cancer. It has broad implications in advancing both cancer biology research and clinical cancer management, including the detection, diagnosis and monitoring of cancer.
Figures

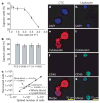


Comment in
-
Cancer diagnostics: one-stop shop.Nature. 2007 Dec 20;450(7173):1168-9. doi: 10.1038/4501168a. Nature. 2007. PMID: 18097389 No abstract available.
References
-
- Cristofanilli M, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. - PubMed
-
- Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nature Med. 2006;12:895–904. - PubMed
-
- Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. - PubMed
-
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. - PubMed
-
- Mocellin S, Hoon D, Ambrosi A, Nitti D, Rossi CR. The prognostic value of circulating tumor cells in patients with melanoma: a systematic review and meta-analysis. Clin Cancer Res. 2006;12:4605–4613. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

