Regulation of UDP-glucuronosyltransferase (UGT) 1A1 by progesterone and its impact on labetalol elimination
- PMID: 18098064
- PMCID: PMC3691104
- DOI: 10.1080/00498250701744633
Regulation of UDP-glucuronosyltransferase (UGT) 1A1 by progesterone and its impact on labetalol elimination
Abstract
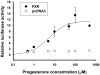
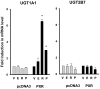
The authors recently reported the increased oral clearance of labetalol in pregnant women. To elucidate the mechanism of the elevated oral clearance, it was hypothesized that female hormones, at the high concentrations attainable during pregnancy, enhance hepatic metabolism of labetalol. Labetalol glucuronidation, which is the major elimination pathway of labetalol, was characterized by screening six recombinant human UGTs (UGT1A1, 1A4, 1A6, 1A9, 2B4, and 2B7) for their capacity to catalyse labetalol glucuronidation. The effect of female hormones (progesterone, oestradiol, oestriol, or oestrone) on the promoter activities of relevant UDP glucuronosyltransferases (UGT) was investigated using a luciferase reporter assay in HepG2 cells. The involvement of oestrogen receptor alpha (ERalpha) and pregnane X receptor (PXR) was examined by co-transfecting ERalpha- or PXR-constructs. UGT1A1 and UGT2B7 were identified as the major UGT enzymes producing labetalol glucuronides (trace amount of glucuronide conjugate was formed by UGT1A9). The activities of the UGT1A1 promoter containing PXR response elements were enhanced by progesterone, but not by oestrogens, indicating PXR-mediated induction of UGT1A1 promoter activity by progesterone. Results from semi-quantitative real-time polymerase chain reaction (PCR) assays are consistent with the above findings. This effect of progesterone on UGT1A1 promoter activities was concentration dependent. Promoter activities of UGT2B7 were not affected by either oestrogens or progesterone. The results suggest a potential role for progesterone in regulating labetalol elimination by modulating the expression of UGT1A1, leading to enhanced drug metabolism during pregnancy.
Figures


Similar articles
-
Pregnancy-Related Hormones Increase UGT1A1-Mediated Labetalol Metabolism in Human Hepatocytes.Front Pharmacol. 2021 Apr 15;12:655320. doi: 10.3389/fphar.2021.655320. eCollection 2021. Front Pharmacol. 2021. PMID: 33995076 Free PMC article.
-
Contribution of UDP-glucuronosyltransferases 1A9 and 2B7 to the glucuronidation of indomethacin in the human liver.Eur J Clin Pharmacol. 2007 Mar;63(3):289-96. doi: 10.1007/s00228-007-0261-0. Epub 2007 Jan 24. Eur J Clin Pharmacol. 2007. PMID: 17245571
-
Human liver UDP-glucuronosyltransferase isoforms involved in the glucuronidation of 7-ethyl-10-hydroxycamptothecin.Xenobiotica. 2001 Oct;31(10):687-99. doi: 10.1080/00498250110057341. Xenobiotica. 2001. PMID: 11695848
-
Genetic polymorphisms of UDP-glucuronosyltransferases and their functional significance.Toxicology. 2002 Dec 27;181-182:453-6. doi: 10.1016/s0300-483x(02)00449-3. Toxicology. 2002. PMID: 12505351 Review.
-
Transcriptional regulation of human UDP-glucuronosyltransferase genes.Drug Metab Rev. 2014 Nov;46(4):421-58. doi: 10.3109/03602532.2014.973037. Epub 2014 Oct 22. Drug Metab Rev. 2014. PMID: 25336387 Review.
Cited by
-
CYP2C19 genotype has a major influence on labetalol pharmacokinetics in healthy male Chinese subjects.Eur J Clin Pharmacol. 2013 Apr;69(4):799-806. doi: 10.1007/s00228-012-1428-x. Epub 2012 Oct 23. Eur J Clin Pharmacol. 2013. PMID: 23090703 Clinical Trial.
-
Influence of gestational diabetes mellitus on the stereoselective kinetic disposition and metabolism of labetalol in hypertensive patients.Eur J Clin Pharmacol. 2011 Jan;67(1):55-61. doi: 10.1007/s00228-010-0896-0. Epub 2010 Sep 17. Eur J Clin Pharmacol. 2011. PMID: 20848091 Clinical Trial.
-
Altered drug metabolism during pregnancy: hormonal regulation of drug-metabolizing enzymes.Expert Opin Drug Metab Toxicol. 2010 Jun;6(6):689-99. doi: 10.1517/17425251003677755. Expert Opin Drug Metab Toxicol. 2010. PMID: 20367533 Free PMC article. Review.
-
Anatomical and physiological alterations of pregnancy.J Pharmacokinet Pharmacodyn. 2020 Aug;47(4):271-285. doi: 10.1007/s10928-020-09677-1. Epub 2020 Feb 6. J Pharmacokinet Pharmacodyn. 2020. PMID: 32026239 Free PMC article. Review.
-
Influence of gestational age and body weight on the pharmacokinetics of labetalol in pregnancy.Clin Pharmacokinet. 2014 Apr;53(4):373-83. doi: 10.1007/s40262-013-0123-0. Clin Pharmacokinet. 2014. PMID: 24297680 Free PMC article.
References
-
- Abernethy DR, Divoll M, Ochs HR, Ameer B, Greenblatt DJ. Increased metabolic clearance of acetaminophen with oral contraceptive use. Obstet Gynecol. 1982;60(3):338–41. - PubMed
-
- Anderson GD. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet. 2005;44(10):989–1008. - PubMed
-
- Balogh A, Klinger G, Henschel L, Borner A, Vollanth R, Kuhnz W. Influence of ethinylestradiol-containing combination oral contraceptives with gestodene or levonorgestrel on caffeine elimination. Eur J Clin Pharmacol. 1995;48(2):161–6. - PubMed
-
- Barkhem T, Haldosen LA, Gustafsson JA, Nilsson S. pS2 Gene expression in HepG2 cells: complex regulation through crosstalk between the estrogen receptor alpha, an estrogen-responsive element, and the activator protein 1 response element. Mol Pharmacol. 2002;61(6):1273–83. - PubMed
-
- Beaulac-Baillargeon L, Rocheleau S. Paracetamol pharmacokinetics during the first trimester of human pregnancy. Eur J Clin Pharmacol. 1994;46(5):451–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
