S-adenosylmethionine prevents Mallory Denk body formation in drug-primed mice by inhibiting the epigenetic memory
- PMID: 18098314
- PMCID: PMC2874456
- DOI: 10.1002/hep.22029
S-adenosylmethionine prevents Mallory Denk body formation in drug-primed mice by inhibiting the epigenetic memory
Abstract
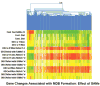
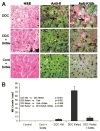
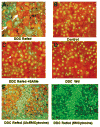
In previous studies, microarray analysis of livers from mice fed diethyl-1,4-dihydro-2,4,6-trimethyl-3,5-pyridine decarboxylate (DDC) for 10 weeks followed by 1 month of drug withdrawal (drug-primed mice) and then 7 days of drug refeeding showed an increase in the expression of numerous genes referred to here as the molecular cellular memory. This memory predisposes the liver to Mallory Denk body formation in response to drug refeeding. In the current study, drug-primed mice were refed DDC with or without a daily dose of S-adenosylmethionine (SAMe; 4 g/kg of body weight). The livers were studied for evidence of oxidative stress and changes in gene expression with microarray analysis. SAMe prevented Mallory Denk body formation in vivo. The molecular cellular memory induced by DDC refeeding lasted for 4 months after drug withdrawal and was not manifest when SAMe was added to the diet in the in vivo experiment. Liver cells from drug-primed mice spontaneously formed Mallory Denk bodies in primary tissue cultures. SAMe prevented Mallory Denk bodies when it was added to the culture medium.
Conclusion: SAMe treatment prevented Mallory Denk body formation in vivo and in vitro by preventing the expression of a molecular cellular memory induced by prior DDC feeding. No evidence for the involvement of oxidative stress in induction of the memory was found. The molecular memory included the up-regulation of the expression of genes associated with the development of liver cell preneoplasia.
Conflict of interest statement
Potential conflict of interest: Nothing to report.
Figures






References
-
- Zatloukal K, French SW, Stumptner C, Strnad P, Harada M, Toivola DM, et al. From Mallory to Mallory-Denk bodies: what, how and why? Exp Cell Res. 2007;313:2033–2049. - PubMed
-
- French BA, van Leeuwen F, Riley NE, Yuan QX, Bardag-Gorce F, Lue YH. Aggresome formation in liver cells in response to different toxic mechanisms. Role of the ubiquitin-proteasome pathway and the frameshift mutant of ubiquitin. Exp Mol Pathol. 2001;71:241–246. - PubMed
-
- Riley NE, Li J, McPhaul L, Lue Y, French SW. Heat shock proteins and Mallory bodies (cytokeratin aggresomes) in human liver biopsies. Exp Mol Pathol. 2003;74:168–174. - PubMed
-
- Zhang-Gouillon ZQ, Yuan QX, Hu B, Gaal K, Marceau N, French SW. Alcohol induces Mallory body formation in drug-primed mice. Hepatology. 1998;27:116–122. - PubMed
-
- Yuan Q, Nagao Y, Wan Y, Hu B, Gaal K, French BA, et al. Mechanisms of Mallory body formation induced by okadaic acid in drug primed mice. Exp Mol Pathol. 1998;65:87–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
