The histamine N-methyltransferase T105I polymorphism affects active site structure and dynamics
- PMID: 18154359
- PMCID: PMC2905460
- DOI: 10.1021/bi701737f
The histamine N-methyltransferase T105I polymorphism affects active site structure and dynamics
Abstract
Histamine N-methyltransferase (HNMT) is the primary enzyme responsible for inactivating histamine in the mammalian brain. The human HNMT gene contains a common threonine-isoleucine polymorphism at residue 105, distal from the active site. The 105I variant has decreased activity and lower protein levels than the 105T protein. Crystal structures of both variants have been determined but reveal little regarding how the T105I polymorphism affects activity. We performed molecular dynamics simulations for both 105T and 105I at 37 degrees C to explore the structural and dynamic consequences of the polymorphism. The simulations indicate that replacing Thr with the larger Ile residue leads to greater burial of residue 105 and heightened intramolecular interactions between residue 105 and residues within helix alpha3 and strand beta3. This altered, tighter packing is translated to the active site, resulting in the reorientation of several cosubstrate-binding residues. The simulations also show that the hydrophobic histamine-binding domain in both proteins undergoes a large-scale breathing motion that exposes key catalytic residues and lowers the hydrophobicity of the substrate-binding site.
Figures


 for α-helices, and
for α-helices, and  for β-strands, and are colored to match the SAM- and histamine-binding domains shown in Figure 1. * = residue 105.
for β-strands, and are colored to match the SAM- and histamine-binding domains shown in Figure 1. * = residue 105.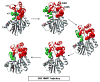
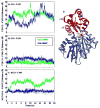

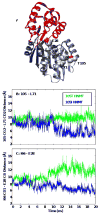
References
-
- Rosenwasser L. New insights into the pathophysiology of allergic rhinitis. Allergy Asthma Proc. 2007;28:10–5. - PubMed
-
- Zhang M, Thurmond RL, Dunford PJ. The histamine H(4) receptor: a novel modulator of inflammatory and immune disorders. Pharmacol Ther. 2007;113:594–606. - PubMed
-
- Schubert ML. Regulation of gastric acid secretion. Curr Opin Gastroenterol. 1999;15:457. - PubMed
-
- Chen D, Friis-Hansen L, Hakanson R, Zhao CM. Genetic dissection of the signaling pathways that control gastric acid secretion. Inflammopharmacology. 2005;13:201–7. - PubMed
-
- Bacciottini L, Passani MB, Mannaioni PF, Blandina P. Interactions between histaminergic and cholinergic systems in learning and memory. Behav Brain Res. 2001;124:183–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

