Pharmacokinetics of an indinavir-ritonavir-fosamprenavir regimen in patients with human immunodeficiency virus
- PMID: 18154477
- PMCID: PMC3073489
- DOI: 10.1592/phco.28.1.74
Pharmacokinetics of an indinavir-ritonavir-fosamprenavir regimen in patients with human immunodeficiency virus
Abstract
Study objective: To evaluate the pharmacokinetic compatibility of a ritonavir-boosted indinavir-fosamprenavir combination among patients with human immunodeficiency virus (HIV).
Design: Single-center, nonrandomized, prospective, multiple-dose, two-phase pharmacokinetic study.
Setting: University research center.
Patients: Eight adult patients with HIV infection who had been receiving and tolerating indinavir 800 mg-ritonavir 100 mg twice/day for at least 2 weeks. Intervention. After 12-hour pharmacokinetic sampling was performed on all patients (period A), fosamprenavir (a prodrug of amprenavir) 700 mg twice/day was coadministered for 5 days, with a repeat 12-hour pharmacokinetic sampling performed on the fifth day (period B).
Measurements and main results: Pharmacokinetic parameters were determined for indinavir, ritonavir, and amprenavir: area under the concentration-time curve from time 0 to 12 hours after dosing (AUC(0-12)), maximum plasma concentration (C(max)), and 12-hour plasma concentration (C(12)). For each parameter, the geometric mean, as well as the geometric mean ratio (GMR) comparing period B with period A, were calculated. Indinavir C(max) was lowered by 20% (GMR 0.80, 95% confidence interval [CI] 0.67-0.96), AUC(0-12) was lowered by 6% (GMR 0.94, 95% CI 0.74-1.21), and C(12) was increased by 28% (GMR 1.28, 95% CI 0.78-2.10). Ritonavir AUC(0-12) was 20% lower (GMR 0.80, 95% CI 0.54-1.19), C(max) was 15% lower (GMR 0.85, 95% CI 0.55-1.32), and C(12) was 7% lower (GMR 0.93, 95% CI 0.49-1.76). With the exception of indinavir C(max), the changes in indinavir and ritonavir pharmacokinetic parameters observed after fosamprenavir coadministration were not statistically significant. The geometric means of amprenavir AUC(0-12), C(max), and C(12) were 41,517 ng*hour/ml (95% CI 30,317-56,854 ng*hr/ml), 5572 ng/ml (95% CI 4330-7170 ng/ml), and 2421 ng/ml (95% CI 1578-3712 ng/ml), respectively.
Conclusion: The combination of indinavir 800 mg-ritonavir 100 mg-fosamprenavir 700 mg twice/day appears to be devoid of a clinically significant drug-drug interaction and should be evaluated as an alternative regimen in salvage HIV treatment. This combination may be suitable as part of a background regimen to optimize the therapeutic benefit of newer classes of antiretroviral agents such as the integrase and coreceptor inhibitors in the treatment of multidrug-resistant viruses.
Figures
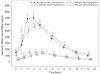
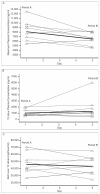
References
-
- Grinsztejn B, Nguyen B, Katlama C, et al. Safety and efficacy of the HIV-1 integrase inhibitor raltegravir (MK-0518) in treatment-experienced patients with multidrug-resistant virus: a phase II randomized controlled trial. Lancet. 2007;369:1261–9. - PubMed
-
- Zolopa AR, Mullen M, Berger D, et al. The HIV integrase inhibitor GS-9137 has potent antiretroviral activity in treatment-experienced patients; Presented at the 14th conference on retroviruses and opportunistic infections; Los Angeles, CA. February 25–28, 2007.
-
- Staszewski S, Babacan E, Stephan C, Stürmer M, Dauer B. The LOPSAQ study: 48-week analysis of a boosted double protease inhibitor regimen containing lopinavir/ritonavir plus saquinavir without additional antiretroviral therapy; Presented at the 16th international AIDS conference; Toronto, Ontario, Canada. August 13–18, 2006. - PubMed
-
- Ribera E, Azuaje C, Lopez R, et al. Atazanavir and lopinavir/ritonavir: pharmacokinetics, safety and efficacy of a promising double-boosted protease inhibitor regimen. AIDS. 2006;20:1131–9. - PubMed
-
- Luber AD. Double-boosted protease inhibitor regimens: a pharmacologic and pharmacokinetic perspective. Clinical care options, HIV, management series, module 1. [Accessed June 14, 2007]. Available from http://www.clinicaloptions.com/HIV/Management%20Series/Double–Boosted%20....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
