Notch2 is required for maintaining sustentacular cell function in the adult mouse main olfactory epithelium
- PMID: 18155189
- PMCID: PMC2374763
- DOI: 10.1016/j.ydbio.2007.10.056
Notch2 is required for maintaining sustentacular cell function in the adult mouse main olfactory epithelium
Abstract
Notch receptors are expressed in neurons and glia in the adult nervous system, but why this expression persists is not well-understood. Here we examine the role of the Notch pathway in the postnatal mouse main olfactory system, and show evidence consistent with a model where Notch2 is required for maintaining sustentacular cell function. In the absence of Notch2, the laminar nature of these glial-like cells is disrupted. Hes1, Hey1, and Six1, which are downstream effectors of the Notch pathway, are down-regulated, and cytochrome P450 and Glutathione S-transferase (GST) expression by sustentacular cells is reduced. Functional levels of GST activity are also reduced. These disruptions are associated with increased olfactory sensory neuron degeneration. Surprisingly, expression of Notch3 is also down-regulated. This suggests the existence of a feedback loop where expression of Notch3 is initially independent of Notch2, but requires Notch2 for maintained expression. While the Notch pathway has previously been shown to be important for promoting gliogenesis during development, this is the first demonstration that the persistent expression of Notch receptors is required for maintaining glial function in adult.
Figures

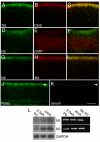
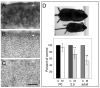





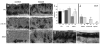
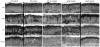

References
-
- Beatus P, Lundkvist J, Oberg C, Lendahl U. The notch 3 intracellular domain represses notch 1-mediated activation through Hairy/Enhancer of split (HES) promoters. Development. 1999;126:3925–35. - PubMed
-
- Berezovska O, Xia MQ, Hyman BT. Notch is expressed in adult brain, is coexpressed with presenilin-1, and is altered in Alzheimer disease. J Neuropathol Exp Neurol. 1998;57:738–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

