Plectin regulates the signaling and trafficking of the HIV-1 co-receptor CXCR4 and plays a role in HIV-1 infection
- PMID: 18155192
- PMCID: PMC2279095
- DOI: 10.1016/j.yexcr.2007.10.032
Plectin regulates the signaling and trafficking of the HIV-1 co-receptor CXCR4 and plays a role in HIV-1 infection
Abstract
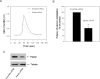
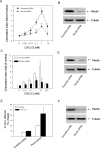
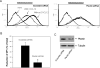
The CXC chemokine CXCL12 and its cognate receptor CXCR4 play an important role in inflammation, human immunodeficiency virus (HIV) infection and cancer metastasis. The signal transduction and intracellular trafficking of CXCR4 are involved in these functions, but the underlying mechanisms remain incompletely understood. In the present study, we demonstrated that the CXCR4 formed a complex with the cytolinker protein plectin in a ligand-dependent manner in HEK293 cells stably expressing CXCR4. The glutathione-S-transferase (GST)-CXCR4 C-terminal fusion proteins co-precipitated with the full-length and the N-terminal fragments of plectin isoform 1 but not with the N-terminal deletion mutants of plectin isoform 1, thereby suggesting an interaction between the N-terminus of plectin and the C-terminus of CXCR4. This interaction was confirmed by confocal microscopic reconstructions showing co-distribution of these two proteins in the internal vesicles after ligand-induced internalization of CXCR4 in HEK293 cells stably expressing CXCR4. Knockdown of plectin with RNA interference (RNAi) significantly inhibited ligand-dependent CXCR4 internalization and attenuated CXCR4-mediated intracellular calcium mobilization and activation of extracellular signal regulated kinase 1/2 (ERK1/2). CXCL12-induced chemotaxis of HEK293 cells stably expressing CXCR4 and of Jurkat T cells was inhibited by the plectin RNAi. Moreover, CXCR4 tropic HIV-1 infection in MAGI (HeLa-CD4-LTR-Gal) cells was inhibited by the RNAi of plectin. Thus, plectin appears to interact with CXCR4 and plays an important role in CXCR4 signaling and trafficking and HIV-1 infection.
Figures
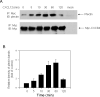
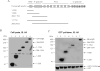
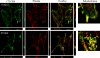
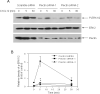

Similar articles
-
Cyclophilin A is required for CXCR4-mediated nuclear export of heterogeneous nuclear ribonucleoprotein A2, activation and nuclear translocation of ERK1/2, and chemotactic cell migration.J Biol Chem. 2008 Jan 4;283(1):623-637. doi: 10.1074/jbc.M704934200. Epub 2007 Nov 8. J Biol Chem. 2008. PMID: 17991743
-
Chemokine CXCL12 induces binding of ferritin heavy chain to the chemokine receptor CXCR4, alters CXCR4 signaling, and induces phosphorylation and nuclear translocation of ferritin heavy chain.J Biol Chem. 2006 Dec 8;281(49):37616-27. doi: 10.1074/jbc.M607266200. Epub 2006 Oct 19. J Biol Chem. 2006. PMID: 17056593
-
HIV-1 clade B Tat, but not clade C Tat, increases X4 HIV-1 entry into resting but not activated CD4+ T cells.J Biol Chem. 2010 Jan 15;285(3):1681-91. doi: 10.1074/jbc.M109.049957. Epub 2009 Nov 16. J Biol Chem. 2010. PMID: 19917610 Free PMC article.
-
CXCL14 antagonizes the CXCL12-CXCR4 signaling axis.Biomol Concepts. 2014 May;5(2):167-73. doi: 10.1515/bmc-2014-0007. Biomol Concepts. 2014. PMID: 25372750 Review.
-
Concise Review: CXCR4/CXCL12 Signaling in Immature Hematopoiesis--Lessons From Pharmacological and Genetic Models.Stem Cells. 2015 Aug;33(8):2391-9. doi: 10.1002/stem.2054. Epub 2015 May 25. Stem Cells. 2015. PMID: 25966814 Review.
Cited by
-
Deubiquitination of CXCR4 by USP14 is critical for both CXCL12-induced CXCR4 degradation and chemotaxis but not ERK ativation.J Biol Chem. 2009 Feb 27;284(9):5742-52. doi: 10.1074/jbc.M808507200. Epub 2008 Dec 23. J Biol Chem. 2009. PMID: 19106094 Free PMC article.
-
Plakin Expression in Serous Epithelial Ovarian Cancer Has the Potential to Impede Metastatic Spread and Epithelial-Mesenchymal Transition: A Comparative Expression Analysis of Immunohistochemical and In Silico Datasets.Cancers (Basel). 2024 Dec 6;16(23):4087. doi: 10.3390/cancers16234087. Cancers (Basel). 2024. PMID: 39682273 Free PMC article.
-
Mechanics of cell sheets: plectin as an integrator of cytoskeletal networks.Open Biol. 2025 Jan;15(1):240208. doi: 10.1098/rsob.240208. Epub 2025 Jan 29. Open Biol. 2025. PMID: 39875099 Free PMC article. Review.
-
Analysis of Pelagia noctiluca proteome Reveals a Red Fluorescent Protein, a Zinc Metalloproteinase and a Peroxiredoxin.Protein J. 2017 Apr;36(2):77-97. doi: 10.1007/s10930-017-9695-0. Protein J. 2017. PMID: 28258523
-
Analysis of Sigma-1 Receptor Antagonist BD1047 Effect on Upregulating Proteins in HIV-1-Infected Macrophages Exposed to Cocaine Using Quantitative Proteomics.Biomedicines. 2024 Aug 23;12(9):1934. doi: 10.3390/biomedicines12091934. Biomedicines. 2024. PMID: 39335448 Free PMC article.
References
-
- Murphy PM. International Union of Pharmacology. XXX. Update on chemokine receptor nomenclature. Pharmacol. Rev . 2002;54:227–229. - PubMed
-
- Dragic T. An overview of the determinants of CCR5 and CXCR4 co-receptor function. J. Gen. Virol. 2001;82:1807–1814. - PubMed
-
- Maekawa T, Ishii T. Chemokine/receptor dynamics in the regulation of hematopoiesis. Intern. Med. 2000;39:90–100. - PubMed
-
- Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. - PubMed
-
- Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S, Kishimoto T, Nagasawa T. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

