Enantiospecific gas chromatographic-mass spectrometric analysis of urinary methylphenidate: implications for phenotyping
- PMID: 18155648
- PMCID: PMC2350184
- DOI: 10.1016/j.jchromb.2007.11.030
Enantiospecific gas chromatographic-mass spectrometric analysis of urinary methylphenidate: implications for phenotyping
Abstract
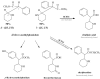
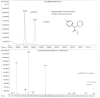
A chiral derivatization gas chromatographic-mass spectrometric (GC-MS) method for urine methylphenidate (MPH) analysis was developed and validated to investigate preliminary findings regarding a novel MPH poor metabolizer (PM). Detection was by electron impact (EI) ionization-selected ion monitoring of the N-trifluoroacetylprolylpiperidinium fragments from MPH and the piperidine-deuterated MPH internal standard. The PM eliminated approximately 70 times more l-MPH in urine (9% of the dose over 0-10h), and approximately 5 times more of the d-isomer (10% of the dose), than the mean values determined from 10 normal metabolizers of MPH. Only minor amounts of the metabolite p-hydroxy-MPH were found in the urine of both the PM and normal metabolizers, while the concentration of MPH lactam was not high enough to be detectable. The described method indirectly gauges the functional carboxylesterase-1 status of patients receiving MPH based on the evaluation of relative urine concentrations of d-MPH:l-MPH. Clinical implications concerning rational drug selection for an identified or suspected MPH PM are discussed.
Figures




References
-
- Biederman J, Spencer T. J Atten Disord. 2002;6(Suppl 1):S101. - PubMed
-
- Markowitz JS, Straughn AB, Patrick KS. Pharmacotherapy. 2003;23:1281. - PubMed
-
- Patrick KS. Drugs. 2006;66:669.
-
- Modi NB, Wang B, Noveck RJ, Gupta SK. J Clin Pharmacol. 2000;40:1141. - PubMed
-
- Patrick KS, Gonzalez MA, Straughn AB, Markowitz JS. Expert Opin Drug Deliv. 2005;2:121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

