Nonequilibrium thermodynamics of thiol/disulfide redox systems: a perspective on redox systems biology
- PMID: 18155672
- PMCID: PMC2587159
- DOI: 10.1016/j.freeradbiomed.2007.11.008
Nonequilibrium thermodynamics of thiol/disulfide redox systems: a perspective on redox systems biology
Abstract
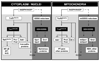
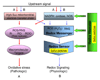
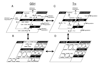
Understanding the dynamics of redox elements in biologic systems remains a major challenge for redox signaling and oxidative stress research. Central redox elements include evolutionarily conserved subsets of cysteines and methionines of proteins which function as sulfur switches and labile reactive oxygen species (ROS) and reactive nitrogen species (RNS) which function in redox signaling. The sulfur switches depend on redox environments in which rates of oxidation are balanced with rates of reduction through the thioredoxins, glutathione/glutathione disulfide, and cysteine/cystine redox couples. These central couples, which we term redox control nodes, are maintained at stable but nonequilibrium steady states, are largely independently regulated in different subcellular compartments, and are quasi-independent from each other within compartments. Disruption of the redox control nodes can differentially affect sulfur switches, thereby creating a diversity of oxidative stress responses. Systems biology provides approaches to address the complexity of these responses. In the present review, we summarize thiol/disulfide pathway, redox potential, and rate information as a basis for kinetic modeling of sulfur switches. The summary identifies gaps in knowledge especially related to redox communication between compartments, definition of redox pathways, and discrimination between types of sulfur switches. A formulation for kinetic modeling of GSH/GSSG redox control indicates that systems biology could encourage novel therapeutic approaches to protect against oxidative stress by identifying specific redox-sensitive sites which could be targeted for intervention.
Figures


References
-
- Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free radical biology & medicine. 2001;30:1191–1212. - PubMed
-
- Hamdane D, Kiger L, Dewilde S, Green BN, Pesce A, Uzan J, Burmester T, Hankeln T, Bolognesi M, Moens L, Marden MC. The redox state of the cell regulates the ligand binding affinity of human neuroglobin and cytoglobin. The Journal of biological chemistry. 2003;278:51713–51721. - PubMed
-
- Jordan PA, Gibbins JM. Extracellular disulfide exchange and the regulation of cellular function. Antioxidants & redox signaling. 2006;8:312–324. - PubMed
-
- Reeves JP, Bailey CA, Hale CC. Redox modification of sodium-calcium exchange activity in cardiac sarcolemmal vesicles. The Journal of biological chemistry. 1986;261:4948–4955. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

