A spatial bias for the origins of interneuron subgroups within the medial ganglionic eminence
- PMID: 18155689
- PMCID: PMC2727678
- DOI: 10.1016/j.ydbio.2007.11.018
A spatial bias for the origins of interneuron subgroups within the medial ganglionic eminence
Abstract
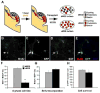
Although it is well established that the ventral telencephalon is the primary source of GABAergic cortical interneurons in rodents, little is known about the specification of specific interneuron subtypes. It is also unclear whether the potential to achieve a given fate is established at their place of origin or by signals received during their migration to or during their maturation within the cerebral cortex. Using both in vivo and in vitro transplantation techniques, we find that two major interneuron subgroups have largely distinct origins within the MGE. Somatostatin (SST)-expressing interneurons are primarily generated within the dorsal MGE, while parvalbumin (PV)-expressing interneurons primarily originate from the ventral MGE. In addition, we show that significant heterogeneity exists between gene expression patterns in the dorsal and ventral MGE. These results suggest that, like the spinal cord, neuronal fate determination in the ventral telencephalon is largely the result of spatially segregated, molecularly distinct microdomains arranged on the dorsal-ventral axis.
Figures




References
-
- Anderson SA, Marin O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–63. - PubMed
-
- Angevine JB, Sidman RL. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature. 1961;192:766–768. - PubMed
-
- Briscoe J, Chen Y, Jessell TM, Struhl G. A hedgehog-insensitive form of patched provides evidence for direct long-range morphogen activity of sonic hedgehog in the neural tube. Mol Cell. 2001;7:1279–91. - PubMed
-
- Butt SJ, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin JG, Fishell G. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

