Effects of selective modulation of the central melanocortin-3-receptor on food intake and hypothalamic POMC expression
- PMID: 18155809
- PMCID: PMC2278043
- DOI: 10.1016/j.peptides.2007.11.005
Effects of selective modulation of the central melanocortin-3-receptor on food intake and hypothalamic POMC expression
Abstract
Hypothalamic POMC neurons regulate energy balance via interactions with brain melanocortin receptors (MC-Rs). POMC neurons express the MC3-R which can function as an inhibitory autoreceptor in vitro. We now demonstrate that central activation of MC3-R with ICV infusion of the specific MC3-R agonist, [D-Trp(8)]-gamma-MSH, transiently suppresses hypothalamic Pomc expression and stimulates food intake in rats. Conversely, we also show that ICV infusion of a low dose of a selective MC3-R antagonist causes a transient decrease in feeding and weight gain. These data support a functional inhibitory role for the MC3-R on POMC neurons that leads to changes in food intake.
Figures
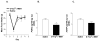
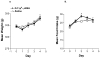
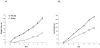
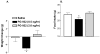
References
-
- Abbott CR, Rossi M, Kim MS, AlAhmed SH, Taylor GM, Ghatei MA, et al. Investigation of the melanocyte stimulating hormones on food intake: lack of evidence to support a role for the melanocortin-3-receptor. Brain Research. 2000;869:203–210. - PubMed
-
- Blum M, Roberts JL, Wardlaw S. Androgen regulation of proopiomelanocortin gene expression and peptide content in the basal hypothalamus. Endocrinology. 1989;124:2283–2288. - PubMed
-
- Bouret S, Prevot V, Croix D, Jegou S, Vaudry H, Stefano GB, et al. Mu-opioid receptor mRNA expression in proopiomelanocortin neurons of the rat arcuate nucleus. Brain Res Mol Brain Res. 1999;70:155–158. - PubMed
-
- Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, et al. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141:3518–3521. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

