Encoding of temporal features of auditory stimuli in the medial nucleus of the trapezoid body and superior paraolivary nucleus of the rat
- PMID: 18155850
- PMCID: PMC2267689
- DOI: 10.1016/j.neuroscience.2007.11.008
Encoding of temporal features of auditory stimuli in the medial nucleus of the trapezoid body and superior paraolivary nucleus of the rat
Abstract
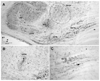
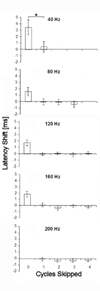
Neurons in the superior paraolivary nucleus (SPON) of the rat respond to the offset of pure tones with a brief burst of spikes. Medial nucleus of the trapezoid body (MNTB) neurons, which inhibit the SPON, produce a sustained pure tone response followed by an offset response characterized by a period of suppressed spontaneous activity. This MNTB offset response is duration dependent and critical to the formation of SPON offset spikes [Kadner A, Kulesza RJ Jr, Berrebi AS (2006) Neurons in the medial nucleus of the trapezoid body and superior paraolivary nucleus of the rat may play a role in sound duration coding. J Neurophysiol. 95:1499-1508; Kulesza RJ Jr, Kadner A, Berrebi AS (2007) Distinct roles for glycine and GABA in shaping the response properties of neurons in the superior paraolivary nucleus of the rat. J Neurophysiol 97:1610-1620]. Here we examine the temporal resolution of the rat's MNTB/SPON circuit by assessing its capability to i) detect gaps in tones, and ii) synchronize to sinusoidally amplitude modulated (SAM) tones. Gap detection was tested by presenting two identical pure tone markers interrupted by gaps ranging from 0 to 25 ms duration. SPON neurons responded to the offset of the leading marker even when the two markers were separated only by their ramps (i.e. a 0 ms gap); longer gap durations elicited progressively larger responses. MNTB neurons produced an offset response at gap durations of 2 ms or longer, with a subset of neurons responding to 0 ms gaps. SAM tone stimuli used the unit's characteristic frequency as a carrier, and modulation rates ranged from 40 to 1160 Hz. MNTB neurons synchronized to modulation rates up to approximately 1 kHz, whereas spiking of SPON neurons decreased sharply at modulation rates >or=400 Hz. Modulation transfer functions based on spike count were all-pass for MNTB neurons and low-pass for SPON neurons; the modulation transfer functions based on vector strength were low-pass for both nuclei, with a steeper cutoff for SPON neurons. Thus, the MNTB/SPON circuit encodes episodes of low stimulus energy, such as gaps in pure tones and troughs in amplitude modulated tones. The output of this circuit consists of brief SPON spiking episodes; their potential effects on the auditory midbrain and forebrain are discussed.
Figures









References
-
- Adams JC. Cytology of periolivary cells and the organization of their projections in the cat. J. Comp. Neurol. 1983;215:275–289. - PubMed
-
- Allen PD, Burkard RF, Ison JR, Walton JP. Impaired gap encoding in aged mouse inferior colliculus at moderate but not high stimulus levels. Hear. Res. 2003;186:17–29. - PubMed
-
- Barsz K, Ison JR, Snell KB, Walton JP. Behavioral and neural measures of auditory temporal acuity in aging humans and mice. Neurobiol. Aging. 2002;23:565–578. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

