Severe intrauterine growth restriction pregnancies have increased placental endoglin levels: hypoxic regulation via transforming growth factor-beta 3
- PMID: 18156205
- PMCID: PMC2189618
- DOI: 10.2353/ajpath.2008.070640
Severe intrauterine growth restriction pregnancies have increased placental endoglin levels: hypoxic regulation via transforming growth factor-beta 3
Abstract
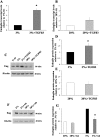
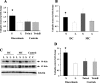
Endoglin, a co-receptor for transforming growth factor (TGF)-beta 1 and -beta 3 is expressed in the human placenta and plays an important role in the pathogenesis of preeclampsia. Because preeclampsia is associated with hypoxia, and because TGF-beta 3 is overexpressed in preeclamptic pregnancies, we examined the effect of oxygen and TGF-beta 3 on placental endoglin expression and investigated its expression in pathological models of placental hypoxia such as intrauterine growth restriction (IUGR) pregnancies. Endoglin expression was high at 4 to 9 weeks of gestation, when oxygen tension is low, and decreased after 10 weeks, when oxygen tension increases. Exposure of villous explants to low oxygen (3% O2) resulted in elevated expression of both membrane and soluble endoglin compared to standard conditions (20% O2). Moreover, addition of TGF-beta 3 to villous explants under low oxygen conditions increased the expression of endoglin compared to nontreated explants whereas addition of TGF-beta 3-neutralizing antibodies inhibited the low oxygen stimulatory effect on endoglin expression. Endoglin and soluble endoglin expression were significantly increased in placentas of IUGR singletons compared to controls and in the IUGR twin placentas relative to both the control co-twin and the normal twins. These data demonstrate that oxygen regulates the placental expression of endoglin via TGF-beta 3. Reduced placental perfusion leading to placental hypoxia might contribute to the increased expression of endoglin in IUGR pregnancies.
Figures

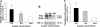


References
-
- Cetin I, Foidart JM, Miozzo M, Raun T, Jansson T, Tsatsaris V, Reik W, Cross J, Hauguel-de-Mouzon S, Illsley N, Kingdom J, Huppertz B. Fetal growth restriction: a workshop report. Placenta. 2004;25:753–757. - PubMed
-
- Pollack RN, Divon MY. Intrauterine growth retardation: definition, classification, and etiology. Clin Obstet Gynecol. 1992;35:99–107. - PubMed
-
- Krebs C, Macara LM, Leiser R, Bowman AW, Greer IA, Kingdom JC. Intrauterine growth restriction with absent end-diastolic flow velocity in the umbilical artery is associated with maldevelopment of the placental terminal villous tree. Am J Obstet Gynecol. 1996;175:1534–1542. - PubMed
-
- Roh CR, Budhraja V, Kim HS, Nelson DM, Sadovsky Y. Microarray-based identification of differentially expressed genes in hypoxic term human trophoblasts and in placental villi of pregnancies with growth restricted fetuses. Placenta. 2005;26:319–328. - PubMed
-
- McCarthy C, Cotter FE, McElwaine S, Twomey A, Mooney EE, Ryan F, Vaughan J. Altered gene expression patterns in intrauterine growth restriction: potential role of hypoxia. Am J Obstet Gynecol. 2007;196:70.e1–e6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

