Prolactin drives estrogen receptor-alpha-dependent ductal expansion and synergizes with transforming growth factor-alpha to induce mammary tumors in males
- PMID: 18156207
- PMCID: PMC2189634
- DOI: 10.2353/ajpath.2008.070597
Prolactin drives estrogen receptor-alpha-dependent ductal expansion and synergizes with transforming growth factor-alpha to induce mammary tumors in males
Abstract
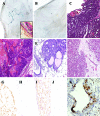
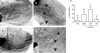
Male breast cancer is rare and has been the focus of limited research. Although the etiology is unclear, conditions increasing circulating prolactin (PRL), as well as estrogen, increase the risk of tumorigenesis. We modeled exposure to elevated PRL in transgenic mice, using the mammary-selective, estrogen-insensitive promoter neu-related lipocalin (NRL), to drive PRL expression. Male NRL-PRL mice did not develop mammary tumors. However, in cooperation with the well-characterized oncogene transforming growth factor-alpha (TGF-alpha), PRL induced mammary tumors in 100% of male bitransgenic mice. Similar to disease in human males, these tumors expressed variable levels of estrogen receptor-alpha (ER-alpha) and androgen receptors. However, carcinogenesis was not responsive to testicular steroids because castration did not alter latency to tumor development or tumor ER-alpha expression. Interestingly, both NRL-TGF-alpha/PRL and NRL-PRL males demonstrated increased ductal development, which occurred during puberty, similar to female mice. This outgrowth was diminished in NRL-PRL males treated with ICI 182,780, suggesting that PRL enhances ER-mediated growth. Treatment of MCF-7-derived cells with PRL increased phosphorylation of ER-alpha at residues implicated in unliganded ER-alpha activity. Together, these studies suggest that PRL expands the pool of cells susceptible to tumorigenesis, which is then facilitated by PRL and TGF-alpha cross talk. Activation of ER-alpha is one mechanism by which PRL may contribute to breast cancer and points to other therapeutic strategies for male patients.
Figures




References
-
- American Cancer Society Atlanta: American Cancer Society; Cancer Facts and Figures, 2006–2007. 2007
-
- Nahleh ZA. Hormonal therapy for male breast cancer: a different approach for a different disease. Cancer Treat Rev. 2006;32:101–105. - PubMed
-
- Krause W. Male breast cancer—an andrological disease: risk factors and diagnosis. Andrologia. 2004;36:346–354. - PubMed
-
- Giordano SH. A review of the diagnosis and management of male breast cancer. Oncologist. 2005;10:471–479. - PubMed
-
- O’Malley C, Shema S, White E, Glaser S. Incidence of male breast cancer in California, 1988–2000: racial/ethnic variation in 1759 men. Breast Cancer Res Treat. 2005;93:145–150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

