Melanin-based high-throughput screen for L-tyrosine production in Escherichia coli
- PMID: 18156325
- PMCID: PMC2258594
- DOI: 10.1128/AEM.02448-07
Melanin-based high-throughput screen for L-tyrosine production in Escherichia coli
Abstract
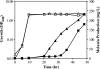
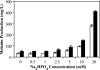
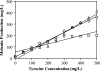
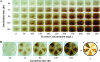
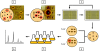
We present the development of a simple, high-throughput screen for identifying bacterial strains capable of L-tyrosine production. Through the introduction of a heterologous gene encoding a tyrosinase, we were able to link L-tyrosine production in Escherichia coli with the synthesis of the black and diffusible pigment melanin. Although melanin was initially produced only at low levels in morpholinepropanesulfonic acid (MOPS) minimal medium, phosphate supplementation was found to be sufficient for increasing both the rates of synthesis and the final titers of melanin. Furthermore, a strong linear correlation between extracellular L-tyrosine content and melanin formation was observed by use of this new medium formulation. A selection strategy that utilizes these findings has been developed and has been shown to be effective in screening large combinatorial libraries in the search for L-tyrosine-overproducing strains.
Figures
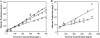

References
-
- Alper, H., K. Miyaoku, and G. Stephanopoulos. 2005. Construction of lycopene-overproducing E. coli strains by combining systematic and combinatorial gene knockout targets. Nat. Biotechnol. 23:612-616. - PubMed
-
- Badarinarayana, V., P. W. Estep III, J. Shendure, J. Edwards, S. Tavazoie, F. Lam, and G. M. Church. 2001. Selection analyses of insertional mutants using subgenic-resolution arrays. Nat. Biotechnol. 19:1060-1065. - PubMed
-
- Bailey, J. E. 1991. Toward a science of metabolic engineering. Science 252:1668-1675. - PubMed
-
- Berry, A. 1996. Improving production of aromatic compounds in Escherichia coli by metabolic engineering. Trends Biotechnol. 14:250-256. - PubMed
-
- Bongaerts, J., M. Kramer, U. Muller, L. Raeven, and M. Wubbolts. 2001. Metabolic engineering for microbial production of aromatic amino acids and derived compounds. Metab. Eng. 3:289-300. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

