Fermentative utilization of glycerol by Escherichia coli and its implications for the production of fuels and chemicals
- PMID: 18156341
- PMCID: PMC2258577
- DOI: 10.1128/AEM.02192-07
Fermentative utilization of glycerol by Escherichia coli and its implications for the production of fuels and chemicals
Abstract
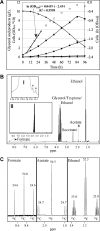
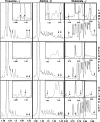
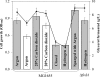
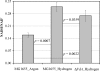
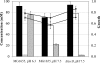
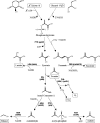
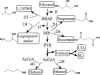
Availability, low prices, and a high degree of reduction make glycerol an ideal feedstock to produce reduced chemicals and fuels via anaerobic fermentation. Although glycerol metabolism in Escherichia coli had been thought to be restricted to respiratory conditions, we report here the utilization of this carbon source in the absence of electron acceptors. Cells grew fermentatively on glycerol and exhibited exponential growth at a maximum specific growth rate of 0.040 +/- 0.003 h(-1). The fermentative nature of glycerol metabolism was demonstrated through studies in which cell growth and glycerol utilization were observed despite blocking several respiratory processes. The incorporation of glycerol in cellular biomass was also investigated via nuclear magnetic resonance analysis of cultures in which either 50% U-13C-labeled or 100% unlabeled glycerol was used. These studies demonstrated that about 20% of the carbon incorporated into the protein fraction of biomass originated from glycerol. The use of U-13C-labeled glycerol also allowed the unambiguous identification of ethanol and succinic, acetic, and formic acids as the products of glycerol fermentation. The synthesis of ethanol was identified as a metabolic determinant of glycerol fermentation; this pathway fulfills energy requirements by generating, in a redox-balanced manner, 1 mol of ATP per mol of glycerol converted to ethanol. A fermentation balance analysis revealed an excellent closure of both carbon (approximately 95%) and redox (approximately 96%) balances. On the other hand, cultivation conditions that prevent H2 accumulation were shown to be an environmental determinant of glycerol fermentation. The negative effect of H2 is related to its metabolic recycling, which in turn generates an unfavorable internal redox state. The implications of our findings for the production of reduced chemicals and fuels were illustrated by coproducing ethanol plus formic acid and ethanol plus hydrogen from glycerol at yields approaching their theoretical maximum.
Figures


References
-
- Bax, A. 1983. A simple method for the calibration of the decoupler radiofrequency field-strength. J. Magn. Res. 52:76-80.
-
- Booth, I. R. March 2005, posting date. Chapter 3.4.3, Glycerol and methylglyoxal metabolism. In R. Curtis III et al. (ed.), EcoSal—Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, DC. http://www.ecosal.org.
-
- Bouvet, O. M. M., P. Lenormand, E. Ageron, and P. A. D. Grimont. 1995. Taxonomic diversity of anaerobic glycerol dissimilation in the Enterobacteriaceae. Res. Microbiol. 146:279-290. - PubMed
-
- Bouvet, O. M. M., P. Lenormand, J. P. Carlier, and P. A. D. Grimont. 1994. Phenotypic diversity of anaerobic glycerol dissimilation shown by 7 enterobacterial species. Res. Microbiol. 145:129-139. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

