The immune response to herpes simplex virus encephalitis in mice is modulated by dietary vitamin E
- PMID: 18156415
- PMCID: PMC2430048
- DOI: 10.1093/jn/138.1.130
The immune response to herpes simplex virus encephalitis in mice is modulated by dietary vitamin E
Abstract
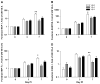
Herpes simplex virus encephalitis (HSE) is the most common fatal sporadic encephalitis in humans. HSE is primarily caused by herpes simplex virus (HSV)-1 infection of the brain. HSE results in increased levels of oxidative stress, including the production of reactive oxygen species, free radicals, and neuroinflammation. The most biologically active form of vitamin E (VE) is alpha-tocopherol (alpha-TOC). In cellular membranes, alpha-TOC prevents lipid peroxidation by scavenging free radicals and functioning as an antioxidant. Supplementation with VE has been shown to decrease immunosenescence, improve immune function, and may be neuroprotective. To determine how VE deficiency and VE supplementation would alter the pathogenesis of HSE, we placed weanling male BALB/cByJ mice on VE-deficient (VE-D), VE-adequate (VE-A), or 10x VE-supplemented diets for 4 wk, and then infected the mice intranasally with HSV-1. VE-D mice had more severe symptoms of encephalitis than VE-A mice, including weight loss, keratitis, hunched posture, and morbidity. VE-D mice had increased cytokine and chemokine expression in the brain and increased viral titers. In contrast, VE supplementation failed to decrease cytokine production and had no effect on viral titer. We demonstrated that adequate levels of VE are important in limiting HSE pathology and that 10x supplementation does not enhance protection.
Conflict of interest statement
Author disclosures: P. A. Sheridan and M. A. Beck, no conflicts of interest.
Figures





References
-
- Johnson M, Valyi-Nagi T. Expanding the clinicopathologic spectrum of herpes simplex encephalitis. Hum Pathol. 1998;29:207–10. - PubMed
-
- Whitley R. Herpes simplex viruses. In: Knipe DM, Howley PM, editors. Fields Virology. 4. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. pp. 2461–509.
-
- Whitley R, Roizman B. Herpes simplex virus infections. Lancet. 2001;357:1513–8. - PubMed
-
- Skoldenberg B. Herpes simplex encephalitis. Scand J Infect Dis Suppl. 1996;100:8–13. - PubMed
-
- Tyler KL. In: Viral meningitis and encephalitis. 15. Braunwald ASFE, Isselbacher KJ, Kasper DL, Hauser SL, Longo DL, Jameson JL, editors. New York: McGraw-Hill; 2002. http://wwwharrisonsonlinecom.

