Cooperative control of striated muscle mass and metabolism by MuRF1 and MuRF2
- PMID: 18157088
- PMCID: PMC2168395
- DOI: 10.1038/sj.emboj.7601952
Cooperative control of striated muscle mass and metabolism by MuRF1 and MuRF2
Abstract
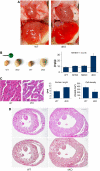
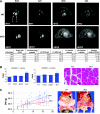
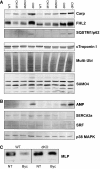
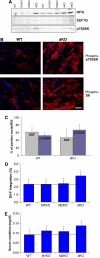
The muscle-specific RING finger proteins MuRF1 and MuRF2 have been proposed to regulate protein degradation and gene expression in muscle tissues. We have tested the in vivo roles of MuRF1 and MuRF2 for muscle metabolism by using knockout (KO) mouse models. Single MuRF1 and MuRF2 KO mice are healthy and have normal muscles. Double knockout (dKO) mice obtained by the inactivation of all four MuRF1 and MuRF2 alleles developed extreme cardiac and milder skeletal muscle hypertrophy. Muscle hypertrophy in dKO mice was maintained throughout the murine life span and was associated with chronically activated muscle protein synthesis. During ageing (months 4-18), skeletal muscle mass remained stable, whereas body fat content did not increase in dKO mice as compared with wild-type controls. Other catabolic factors such as MAFbox/atrogin1 were expressed at normal levels and did not respond to or prevent muscle hypertrophy in dKO mice. Thus, combined inhibition of MuRF1/MuRF2 could provide a potent strategy to stimulate striated muscles anabolically and to protect muscles from sarcopenia during ageing.
Figures


Similar articles
-
Expression of MuRF1 or MuRF2 is essential for the induction of skeletal muscle atrophy and dysfunction in a murine pulmonary hypertension model.Skelet Muscle. 2020 Apr 27;10(1):12. doi: 10.1186/s13395-020-00229-2. Skelet Muscle. 2020. PMID: 32340625 Free PMC article.
-
Muscle ring finger 1 and muscle ring finger 2 are necessary but functionally redundant during developmental cardiac growth and regulate E2F1-mediated gene expression in vivo.Cell Biochem Funct. 2014 Jan;32(1):39-50. doi: 10.1002/cbf.2969. Epub 2013 Mar 20. Cell Biochem Funct. 2014. PMID: 23512667 Free PMC article.
-
MuRF1 is a muscle fiber-type II associated factor and together with MuRF2 regulates type-II fiber trophicity and maintenance.J Struct Biol. 2010 May;170(2):344-53. doi: 10.1016/j.jsb.2010.02.001. Epub 2010 Feb 10. J Struct Biol. 2010. PMID: 20149877 Free PMC article.
-
Molecular regulation of skeletal muscle mass.Clin Exp Pharmacol Physiol. 2010 Mar;37(3):378-84. doi: 10.1111/j.1440-1681.2009.05265.x. Epub 2009 Jul 24. Clin Exp Pharmacol Physiol. 2010. PMID: 19650790 Review.
-
Signalling pathways that mediate skeletal muscle hypertrophy and atrophy.Nat Cell Biol. 2003 Feb;5(2):87-90. doi: 10.1038/ncb0203-87. Nat Cell Biol. 2003. PMID: 12563267 Review.
Cited by
-
MuRF1/TRIM63, Master Regulator of Muscle Mass.Int J Mol Sci. 2020 Sep 11;21(18):6663. doi: 10.3390/ijms21186663. Int J Mol Sci. 2020. PMID: 32933049 Free PMC article. Review.
-
The GSK-3β-FBXL21 Axis Contributes to Circadian TCAP Degradation and Skeletal Muscle Function.Cell Rep. 2020 Sep 15;32(11):108140. doi: 10.1016/j.celrep.2020.108140. Cell Rep. 2020. PMID: 32937135 Free PMC article.
-
Uncovering the mechanisms of MuRF1-induced ubiquitylation and revealing similarities with MuRF2 and MuRF3.Biochem Biophys Rep. 2024 Jan 6;37:101636. doi: 10.1016/j.bbrep.2023.101636. eCollection 2024 Mar. Biochem Biophys Rep. 2024. PMID: 38283190 Free PMC article.
-
The myogenic electric organ of Sternopygus macrurus: a non-contractile tissue with a skeletal muscle transcriptome.PeerJ. 2016 Apr 14;4:e1828. doi: 10.7717/peerj.1828. eCollection 2016. PeerJ. 2016. PMID: 27114860 Free PMC article.
-
Small-Molecule Inhibition of MuRF1 Prevents Early Disuse-Induced Diaphragmatic Dysfunction and Atrophy.Int J Mol Sci. 2023 Feb 11;24(4):3637. doi: 10.3390/ijms24043637. Int J Mol Sci. 2023. PMID: 36835047 Free PMC article.
References
-
- Baar K, Torgan CE, Kraus WE, Esser K (2000) Autocrine phosphorylation of p70(S6k) in response to acute stretch in myotubes. Mol Cell Biol Res Commun 4: 76–80 - PubMed
-
- Bassel-Duby R, Olson EN (2006) Signaling pathways in skeletal muscle remodeling. Annu Rev Biochem 75: 19–37 - PubMed
-
- Belle R, Minella O, Cormier P, Morales J, Poulhe R, Mulner-Lorillon O (1995) Phosphorylation of elongation factor-1 (EF-1) by cdc2 kinase. Prog Cell Cycle Res 1: 265–270 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

