Semiautomated radiosynthesis and biological evaluation of [18F]FEAU: a novel PET imaging agent for HSV1-tk/sr39tk reporter gene expression
- PMID: 18157580
- PMCID: PMC4141558
- DOI: 10.1007/s11307-007-0122-3
Semiautomated radiosynthesis and biological evaluation of [18F]FEAU: a novel PET imaging agent for HSV1-tk/sr39tk reporter gene expression
Abstract
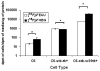
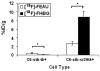
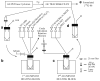
2'-deoxy-2'-[(18)F]fluoro-5-ethyl-1-beta-D-arabinofuranosyluracil ([(18)F]FEAU) is a promising radiolabeled nucleoside designed to monitor Herpes Simplex Virus Type 1 thymidine kinase (HSV1-tk) reporter gene expression with positron emission tomography (PET). However, the challenging radiosynthesis creates problems for being able to provide [(18)F]FEAU routinely. We have developed a routine method using a commercial GE TRACERlab FX-FN radiosynthesis module with customized equipment to provide [(18)F]FEAU. All radiochemical yields are decay corrected to end-of-bombardment and reported as means +/- SD. Radiofluorination (33 +/- 8%; n = 4), bromination (85 +/- 8%; n = 4), coupling reaction (83 +/- 6%; n = 4), base hydrolysis steps, and subsequent high-performance liquid chromatography purification afforded purified [(18)F]FEAU beta-anomer in 5 +/- 1% overall yield (n = 3 runs) after approximately 5.5 h and a beta/alpha-anomer ratio of 7.4. Radiochemical/chemical purities and specific activity exceeded 99% and 1.3 Ci/micromol (48 GBq/micromol), respectively. In cell culture, [(18)F]FEAU showed significantly (P < 0.05) higher accumulation in C6 cells expressing HSV1-tk/sr39tk as compared to wild-type C6 cells. Furthermore, [(18)F]FEAU showed slightly higher accumulation than 9-[4-[(18)F]fluoro-3-(hydroxymethyl)butylguanine ([(18)F]FHBG) in cells expressing HSV1-tk (P < 0.05), whereas [(18)F]FHBG showed significantly higher (P < 0.05) accumulation than [(18)F]FEAU in HSV1-sr39tk-expressing cells. micro-PET imaging of mice carrying tumor xenografts of C6 cells stably expressing HSV1-tk or HSV1-sr39tk are consistent with the cell uptake results. The [(18)F]FEAU mouse images also showed very low gastrointestinal signal with predominant renal clearance as compared to [(18)F]FHBG. The routine radiosynthesis of [(18)F]FEAU was successfully semiautomated using a commercial module along with customized equipment to provide the beta-anomer in modest yields. Although further studies are needed, early results also suggest [(18)F]FEAU is a promising PET radiotracer for monitoring HSV1-tk reporter gene expression.
Figures





References
-
- Penuelas I, Gambhir SS. Imaging studies for evaluating gene therapy in translational research. Drug Discov Today Technol. 2005;2:335–343. - PubMed
-
- Min JJ, Gambhir SS. Gene therapy progress and prospects. Noninvasive imaging of gene therapy in living subjects. Gene Therapy. 2004;11:115–125. - PubMed
-
- Watanabe KA, Reichman U, Hirota K, Lopez C, Fox JJ. Nucleosides. 110, synthesis and antiherpes virus activity of some 2′-fluoro-2′deoxyarabinofuranosylpyrimidine nucleosides. J Med Chem. 1979;22:21–24. - PubMed
-
- Watanabe KA, Su TL, Klein RS, et al. Nucleosides. 123. Synthesis of antiviral nucleosides: 5-substituted of 1-(2-deoxy-2-halogeno-β-D-arabinofuranosyl)cytosines and-uracils. some structure-activity relationships. J Med Chem. 1983;26:152–156. - PubMed
-
- Watanabe KA, Su TL, Reichman U, Greenberg N, Lopez C, Fox JJ. Nucleosides. 129. Synthesis of antiviral nucleosides: 5-alkenyl-1-(2-deoxy-2-fluoro-β-D-arabinofuranosyl)uracils. J Med Chem. 1984;27:91–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

