Reversibility of PRKAG2 glycogen-storage cardiomyopathy and electrophysiological manifestations
- PMID: 18158359
- PMCID: PMC2957811
- DOI: 10.1161/CIRCULATIONAHA.107.726752
Reversibility of PRKAG2 glycogen-storage cardiomyopathy and electrophysiological manifestations
Abstract
Background: PRKAG2 mutations cause glycogen-storage cardiomyopathy, ventricular preexcitation, and conduction system degeneration. A genetic approach that utilizes a binary inducible transgenic system was used to investigate the disease mechanism and to assess preventability and reversibility of disease features in a mouse model of glycogen-storage cardiomyopathy.
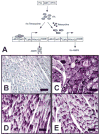
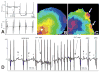
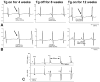
Methods and results: Transgenic (Tg) mice expressing a human N488I PRKAG2 cDNA under control of the tetracycline-repressible alpha-myosin heavy chain promoter underwent echocardiography, ECG, and in vivo electrophysiology studies. Transgene suppression by tetracycline administration caused a reduction in cardiac glycogen content and was initiated either prenatally (Tg(OFF(E-8 weeks))) or at different time points during life (Tg(OFF(4-16 weeks)), Tg(OFF(8-20 weeks)), and Tg(OFF(>20 weeks))). One group never received tetracycline, expressing transgene throughout life (Tg(ON)). Tg(ON) mice developed cardiac hypertrophy followed by dilatation, ventricular preexcitation involving multiple accessory pathways, and conduction system disease, including sinus and atrioventricular node dysfunction.
Conclusions: Using an externally modifiable transgenic system, cardiomyopathy, cardiac dysfunction, and electrophysiological disorders were demonstrated to be reversible processes in PRKAG2 disease. Transgene suppression during early postnatal development prevented the development of accessory electrical pathways but not cardiomyopathy or conduction system degeneration. Taken together, these data provide insight into mechanisms of cardiac PRKAG2 disease and suggest that glycogen-storage cardiomyopathy can be modulated by lowering glycogen content in the heart.
Figures



Similar articles
-
Transgenic mice overexpressing mutant PRKAG2 define the cause of Wolff-Parkinson-White syndrome in glycogen storage cardiomyopathy.Circulation. 2003 Jun 10;107(22):2850-6. doi: 10.1161/01.CIR.0000075270.13497.2B. Epub 2003 Jun 2. Circulation. 2003. PMID: 12782567
-
Electrophysiologic characterization and postnatal development of ventricular pre-excitation in a mouse model of cardiac hypertrophy and Wolff-Parkinson-White syndrome.J Am Coll Cardiol. 2003 Sep 3;42(5):942-51. doi: 10.1016/s0735-1097(03)00850-7. J Am Coll Cardiol. 2003. PMID: 12957447
-
Transgenic knockdown of cardiac sodium/glucose cotransporter 1 (SGLT1) attenuates PRKAG2 cardiomyopathy, whereas transgenic overexpression of cardiac SGLT1 causes pathologic hypertrophy and dysfunction in mice.J Am Heart Assoc. 2014 Aug 4;3(4):e000899. doi: 10.1161/JAHA.114.000899. J Am Heart Assoc. 2014. PMID: 25092788 Free PMC article.
-
Glycogen storage disease as a unifying mechanism of disease in the PRKAG2 cardiac syndrome.Biochem Soc Trans. 2003 Feb;31(Pt 1):228-31. doi: 10.1042/bst0310228. Biochem Soc Trans. 2003. PMID: 12546691 Review.
-
PRKAG2 cardiac syndrome: familial ventricular preexcitation, conduction system disease, and cardiac hypertrophy.Curr Opin Cardiol. 2002 May;17(3):229-34. doi: 10.1097/00001573-200205000-00004. Curr Opin Cardiol. 2002. PMID: 12015471 Review.
Cited by
-
Electrocardiographic Characteristics and Their Correlation with Echocardiographic Alterations in Fabry Disease.J Cardiovasc Dev Dis. 2022 Jan 3;9(1):11. doi: 10.3390/jcdd9010011. J Cardiovasc Dev Dis. 2022. PMID: 35050221 Free PMC article.
-
Wolff-Parkinson-White syndrome: De novo variants and evidence for mutational burden in genes associated with atrial fibrillation.Am J Med Genet A. 2020 Jun;182(6):1387-1399. doi: 10.1002/ajmg.a.61571. Epub 2020 Mar 31. Am J Med Genet A. 2020. PMID: 32233023 Free PMC article.
-
Contribution of hypoxia-inducible factor 1alpha to pathogenesis of sarcomeric hypertrophic cardiomyopathy.Sci Rep. 2025 Jan 16;15(1):2132. doi: 10.1038/s41598-025-85187-9. Sci Rep. 2025. PMID: 39820339 Free PMC article.
-
Revisiting Diagnosis and Treatment of Hypertrophic Cardiomyopathy: Current Practice and Novel Perspectives.J Clin Med. 2023 Sep 1;12(17):5710. doi: 10.3390/jcm12175710. J Clin Med. 2023. PMID: 37685777 Free PMC article. Review.
-
AMP-activated protein kinase: a key regulator of energy balance with many roles in human disease.J Intern Med. 2014 Dec;276(6):543-59. doi: 10.1111/joim.12268. Epub 2014 May 27. J Intern Med. 2014. PMID: 24824502 Free PMC article. Review.
References
-
- Blair E, Redwood C, Ashrafian H, Oliveira M, Broxholme J, Kerr B, Salmon A, Ostman-Smith I, Watkins H. Mutations in the gamma(2) subunit of AMP-activated protein kinase cause familial hypertrophic cardiomyopathy: evidence for the central role of energy compromise in disease pathogenesis. Hum Mol Genet. 2001;10:1215–1220. - PubMed
-
- Arad M, Seidman CE, Seidman JG. AMP-activated protein kinase in the heart: role during health and disease. Circ Res. 2007;100:474–488. - PubMed
-
- Hardie DG, Sakamoto K. AMPK: a key sensor of fuel and energy status in skeletal muscle. Physiology. 2006;21:48–60. - PubMed
-
- Ingwall JS. Transgenesis and cardiac energetics: new insights into cardiac metabolism. J Mol Cell Cardiol. 2004;37:613–623. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

