STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels
- PMID: 18160041
- PMCID: PMC2680164
- DOI: 10.1016/j.cell.2007.11.039
STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels
Abstract
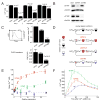
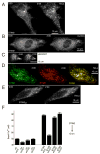
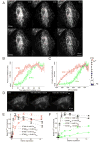
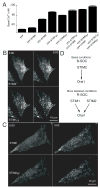
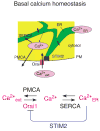
Deviations in basal Ca2+ levels interfere with receptor-mediated Ca2+ signaling as well as endoplasmic reticulum (ER) and mitochondrial function. While defective basal Ca2+ regulation has been linked to various diseases, the regulatory mechanism that controls basal Ca2+ is poorly understood. Here we performed an siRNA screen of the human signaling proteome to identify regulators of basal Ca2+ concentration and found STIM2 as the strongest positive regulator. In contrast to STIM1, a recently discovered signal transducer that triggers Ca2+ influx in response to receptor-mediated depletion of ER Ca2+ stores, STIM2 activated Ca2+ influx upon smaller decreases in ER Ca2+. STIM2, like STIM1, caused Ca2+ influx via activation of the plasma membrane Ca2+ channel Orai1. Our study places STIM2 at the center of a feedback module that keeps basal cytosolic and ER Ca2+ concentrations within tight limits.
Figures


References
-
- Berridge MJ, Bootman MD, Roderick HL. Ca2+ signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–29. - PubMed
-
- Bezprozvanny I, Hayden MR. Deranged neuronal Ca2+ signaling and Huntington disease. Biochem Biophys Res Commun. 2004;322:1310–7. - PubMed
-
- Brini M, Pinton P, Pozzan T, Rizzuto R. Targeted recombinant aequorins: tools for monitoring [Ca2+] in the various compartments of a living cell. Microsc Res Tech. 1999;46:380–9. - PubMed
-
- Camello C, Lomax R, Petersen OH, Tepikin AV. Calcium leak from intracellular stores--the enigma of calcium signalling. Cell Calcium. 2002;32:355–61. - PubMed
-
- Campanella M, Pinton P, Rizzuto R. Mitochondrial Ca2+ homeostasis in health and disease. Biol Res. 2004;37:653–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

