Identifying the site of spin trapping in proteins by a combination of liquid chromatography, ELISA, and off-line tandem mass spectrometry
- PMID: 18160050
- PMCID: PMC2268891
- DOI: 10.1016/j.freeradbiomed.2007.11.015
Identifying the site of spin trapping in proteins by a combination of liquid chromatography, ELISA, and off-line tandem mass spectrometry
Abstract
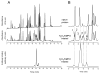
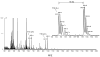
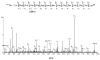
An off-line mass spectrometry method that combines immuno-spin trapping and chromatographic procedures has been developed for selective detection of the nitrone spin trap 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) covalently attached to proteins, an attachment which occurs only subsequent to DMPO trapping of free radicals. In this technique, the protein-DMPO nitrone adducts are digested to peptides with proteolytic agents, peptides from the enzymatic digest are separated by HPLC, and enzyme-linked immunosorbent assays (ELISA) using polyclonal anti-DMPO nitrone antiserum are used to detect the eluted HPLC fractions that contain DMPO nitrone adducts. The fractions showing positive ELISA signals are then concentrated and characterized by tandem mass spectrometry (MS/MS). This method, which constitutes the first liquid chromatography-ELISA-mass spectrometry (LC-ELISA-MS)-based strategy for selective identification of DMPO-trapped protein residues in complex peptide mixtures, facilitates location and preparative fractionation of DMPO nitrone adducts for further structural characterization. The strategy is demonstrated for human hemoglobin, horse heart myoglobin, and sperm whale myoglobin, three globin proteins known to form DMPO-trappable protein radicals on treatment with H(2)O(2). The results demonstrate the power of the new experimental strategy to select DMPO-labeled peptides and identify sites of DMPO covalent attachments.
Figures





References
-
- Stubbe J, van der Donk WA. Protein radicals in enzyme catalysis. Chem Rev. 1998;98:705–762. - PubMed
-
- Dalle-Donne I, Scaloni A, Giustarini D, Cavarra E, Tell G, Lungarella G, Colombo R, Rossi R, Milzani A. Proteins as biomarkers of oxidative/nitrosative stress in diseases: the contribution of redox proteomics. Mass Spec Rev. 2005;24:55–99. - PubMed
-
- Davies MJ, Fu S, Wang H, Dean RT. Stable markers of oxidant damage to proteins and their application in the study of human disease. Free Radic Biol Med. 1999;27:1151–1163. - PubMed
-
- Davies MJ, Hawkins CL. EPR spin trapping of protein radicals. Free Radic BiolMed. 2004;36:1072–1086. - PubMed
-
- Augusto O, Vaz SM. EPR spin-trapping of protein radicals to investigate biological oxidative mechanisms. Amino Acids. 2007;32:535–542. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

