A Presenilin-like protease associated with Plasmodium falciparum micronemes is involved in erythrocyte invasion
- PMID: 18160114
- PMCID: PMC2268099
- DOI: 10.1016/j.molbiopara.2007.11.007
A Presenilin-like protease associated with Plasmodium falciparum micronemes is involved in erythrocyte invasion
Abstract
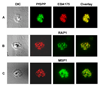
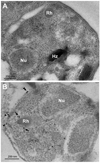
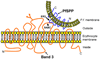
We describe identification of a Plasmodium falciparum microneme protease involved in RBC invasion. From the yeast two-hybrid screening of a P. falciparum cDNA library, we have identified a 47 kDa membrane protein that interacted with the 5ABC domain of human RBC band 3. This protein shared homology with a Presenilin-type aspartyl protease, the signal peptide peptidase (SPP). An antibody raised against a predicted exposed region of this protein reacted specifically to a single band of approximately 47 kDa in the P. falciparum protein extract. Immunofluorescence microscopy suggested that this protein co-localized with the microneme protein EBA-175 in schizonts, and immunoelectron microscopy established that it is primarily localized to micronemes in merozoites. Functional characterization of Plasmodium falciparum signal peptide peptidase (PfSPP), demonstrates that an antibody to PfSPP blocks RBC invasion by P. falciparumin vitro. Native and recombinant PfSPP bound directly to the 5ABC domain of band 3 in solution and the binding of PfSPP to RBCs was chymotrypsin-sensitive, but trypsin and neuraminidase-resistant. Together, these results suggest that host band 3 interacts with PfSPP during RBC invasion presumably following parasite microneme discharge. PfSPP is the first microneme-associated intramembrane aspartyl protease identified in the apicomplexan parasites that interacts with a major transmembrane receptor on host erythrocytes.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

