Apolipoprotein AII is a regulator of very low density lipoprotein metabolism and insulin resistance
- PMID: 18160395
- PMCID: PMC2431075
- DOI: 10.1074/jbc.M708995200
Apolipoprotein AII is a regulator of very low density lipoprotein metabolism and insulin resistance
Abstract
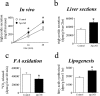
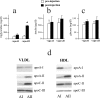
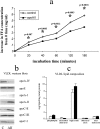
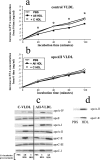
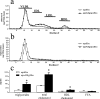
Apolipoprotein AII (apoAII) transgenic (apoAIItg) mice exhibit several traits associated with the insulin resistance (IR) syndrome, including IR, obesity, and a marked hypertriglyceridemia. Because treatment of the apoAIItg mice with rosiglitazone ameliorated the IR and hypertriglyceridemia, we hypothesized that the hypertriglyceridemia was due largely to overproduction of very low density lipoprotein (VLDL) by the liver, a normal response to chronically elevated insulin and glucose. We now report in vivo and in vitro studies that indicate that hepatic fatty acid oxidation was reduced and lipogenesis increased, resulting in a 25% increase in triglyceride secretion in the apoAIItg mice. In addition, we observed that hydrolysis of triglycerides from both chylomicrons and VLDL was significantly reduced in the apoAIItg mice, further contributing to the hypertriglyceridemia. This is a direct, acute effect, because when mouse apoAII was injected into mice, plasma triglyceride concentrations were significantly increased within 4 h. VLDL from both control and apoAIItg mice contained significant amounts of apoAII, with approximately 4 times more apoAII on apoAIItg VLDL. ApoAII was shown to transfer spontaneously from high density lipoprotein (HDL) to VLDL in vitro, resulting in VLDL that was a poorer substrate for hydrolysis by lipoprotein lipase. These results indicate that one function of apoAII is to regulate the metabolism of triglyceride-rich lipoproteins, with HDL serving as a plasma reservoir of apoAII that is transferred to the triglyceride-rich lipoproteins in much the same way as VLDL and chylomicrons acquire most of their apoCs from HDL.
Figures


References
-
- Schonfeld, G., Bailey, A., and Steelman, R. (1978) Lipids 12 951-959 - PubMed
-
- Tall, A. R. (1998) Eur. Heart J. 19 Suppl. A, A31-A35 - PubMed
-
- Barbaras, R., Puchois, P., Fruchart, J. C., and Ailhaud, G. (1987) Biochem. Biophys. Res. Commun. 142 63-69 - PubMed
-
- Warden, C. H., Hedrick, C. C., Qiao, J. H., Castellani, L. W., and Lusis, A. J. (1993) Science 261 469-472 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

