Mechanosensory gating of proprioceptor input to modulatory projection neurons
- PMID: 18160638
- PMCID: PMC6494452
- DOI: 10.1523/JNEUROSCI.4404-07.2007
Mechanosensory gating of proprioceptor input to modulatory projection neurons
Abstract
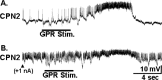
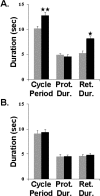
Sensorimotor gating commonly occurs at sensory neuron synapses onto motor circuit neurons and motor neurons. Here, using the crab stomatogastric nervous system, we show that sensorimotor gating also occurs at the level of the projection neurons that activate motor circuits. We compared the influence of the gastro-pyloric receptor (GPR) muscle stretch-sensitive neuron on two projection neurons, modulatory commissural neuron 1 (MCN1) and commissural projection neuron 2 (CPN2), with and without a preceding activation of the mechanosensory ventral cardiac neurons (VCNs). MCN1 and CPN2 project from the paired commissural ganglia (CoGs) to the stomatogastric ganglion (STG), where they activate the gastric mill (chewing) motor circuit. When stimulated separately, the GPR and VCN neurons each elicit the gastric mill rhythm by coactivating MCN1 and CPN2. When GPR is instead stimulated during the VCN-gastric mill rhythm, it slows this rhythm. This effect results from a second GPR synapse onto MCN1 that presynaptically inhibits its STG terminals. Here, we show that, during the VCN-triggered rhythm, the GPR excitation of MCN1 and CPN2 in the CoGs is gated out, leaving only its influence in the STG. This gating effect appears to occur within the CoG and does not result from a ceiling effect on projection neuron firing frequency. Additionally, this gating action enables GPR to either activate rhythmic motor activity or act as a phasic sensorimotor feedback system. These results also indicate that the site of sensorimotor gating can occur at the level of the projection neurons that activate a motor circuit.
Figures


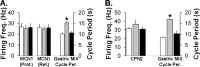




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
