A critical function for beta-amyloid precursor protein in neuronal migration revealed by in utero RNA interference
- PMID: 18160654
- PMCID: PMC6673432
- DOI: 10.1523/JNEUROSCI.4701-07.2007
A critical function for beta-amyloid precursor protein in neuronal migration revealed by in utero RNA interference
Abstract
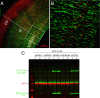
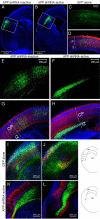
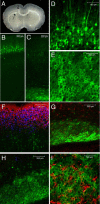
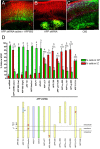
Physiological processing of the beta-amyloid precursor protein (APP) generates amyloid beta-protein, which can assemble into oligomers that mediate synaptic failure in Alzheimer's disease. Two decades of research have led to human trials of compounds that chronically target this processing, and yet the normal function of APP in vivo remains unclear. We used the method of in utero electroporation of shRNA constructs into the developing cortex to acutely knock down APP in rodents. This approach revealed that neuronal precursor cells in embryonic cortex require APP to migrate correctly into the nascent cortical plate. cDNAs encoding human APP or its homologues, amyloid precursor-like protein 1 (APLP1) or APLP2, fully rescued the shRNA-mediated migration defect. Analysis of an array of mutations and deletions in APP revealed that both the extracellular and cytoplasmic domains of APP are required for efficient rescue. Whereas knock-down of APP inhibited cortical plate entry, overexpression of APP caused accelerated migration of cells past the cortical plate boundary, confirming that normal APP levels are required for correct neuronal migration. In addition, we found that Disabled-1 (Dab1), an adaptor protein with a well established role in cortical cell migration, acts downstream of APP for this function in cortical plate entry. We conclude that full-length APP functions as an important factor for proper migration of neuronal precursors into the cortical plate during the development of the mammalian brain.
Figures

References
-
- Anliker B, Muller U. The functions of mammalian amyloid precursor protein and related amyloid precursor-like proteins. Neurodegener Dis. 2006;3:239–246. - PubMed
-
- Araki W, Kitaguchi N, Tokushima Y, Ishii K, Aratake H, Shimohama S, Nakamura S, Kimura J. Trophic effect of β-amyloid precursor protein on cerebral cortical neurons in culture. Biochem Biophys Res Commun. 1991;181:265–271. - PubMed
-
- Bai J, Ramos RL, Ackman JB, Thomas AM, Lee RV, LoTurco JJ. RNAi reveals doublecortin is required for radial migration in rat neocortex. Nat Neurosci. 2003;6:1277–1283. - PubMed
-
- Bayer SA, Altman J. Development of the endopiriform nucleus and the claustrum in the rat brain. Neuroscience. 1991;45:391–412. - PubMed
-
- Bayer SA, Altman J, Russo RJ, Dai XF, Simmons JA. Cell migration in the rat embryonic neocortex. J Comp Neurol. 1991;307:499–516. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
