Infectious Disease: Connecting Innate Immunity to Biocidal Polymers
- PMID: 18160969
- PMCID: PMC2153456
- DOI: 10.1016/j.mser.2007.03.002
Infectious Disease: Connecting Innate Immunity to Biocidal Polymers
Abstract
Infectious disease is a critically important global healthcare issue. In the U.S. alone there are 2 million new cases of hospital-acquired infections annually leading to 90,000 deaths and 5 billion dollars of added healthcare costs. Couple these numbers with the appearance of new antibiotic resistant bacterial strains and the increasing occurrences of community-type outbreaks, and clearly this is an important problem. Our review attempts to bridge the research areas of natural host defense peptides (HDPs), a component of the innate immune system, and biocidal cationic polymers. Recently discovered peptidomimetics and other synthetic mimics of HDPs, that can be short oligomers as well as polymeric macromolecules, provide a unique link between these two areas. An emerging class of these mimics are the facially amphiphilic polymers that aim to emulate the physicochemical properties of HDPs but take advantage of the synthetic ease of polymers. These mimics have been designed with antimicrobial activity and, importantly, selectivity that rivals natural HDPs. In addition to providing some perspective on HDPs, selective mimics, and biocidal polymers, focus is given to the arsenal of biophysical techniques available to study their mode of action and interactions with phospholipid membranes. The issue of lipid type is highlighted and the important role of negative curvature lipids is illustrated. Finally, materials applications (for instance, in the development of permanently antibacterial surfaces) are discussed as this is an important part of controlling the spread of infectious disease.
Figures
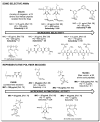
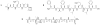
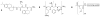
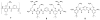
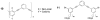
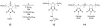
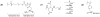
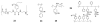

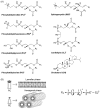
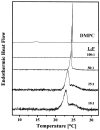
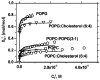


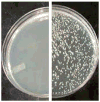


References
-
- Infectious Diseases Society of America report. Alexandria, VA: 2004. Bad Bugs, No Drugs, As Antibiotic Discovery Stagnates, a Public Health Crisis Brews; pp. 1–35. http://www.idsociety.org.
-
- Katchalski E, Bichovski-Slomnitzki L, Volcani BE. Nature. 1952;169:1095–1096. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
