Pediatric measles vaccine expressing a dengue antigen induces durable serotype-specific neutralizing antibodies to dengue virus
- PMID: 18160988
- PMCID: PMC2154386
- DOI: 10.1371/journal.pntd.0000096
Pediatric measles vaccine expressing a dengue antigen induces durable serotype-specific neutralizing antibodies to dengue virus
Abstract
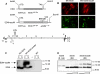
Dengue disease is an increasing global health problem that threatens one-third of the world's population. Despite decades of efforts, no licensed vaccine against dengue is available. With the aim to develop an affordable vaccine that could be used in young populations living in tropical areas, we evaluated a new strategy based on the expression of a minimal dengue antigen by a vector derived from pediatric live-attenuated Schwarz measles vaccine (MV). As a proof-of-concept, we inserted into the MV vector a sequence encoding a minimal combined dengue antigen composed of the envelope domain III (EDIII) fused to the ectodomain of the membrane protein (ectoM) from DV serotype-1. Immunization of mice susceptible to MV resulted in a long-term production of DV1 serotype-specific neutralizing antibodies. The presence of ectoM was critical to the immunogenicity of inserted EDIII. The adjuvant capacity of ectoM correlated with its ability to promote the maturation of dendritic cells and the secretion of proinflammatory and antiviral cytokines and chemokines involved in adaptive immunity. The protective efficacy of this vaccine should be studied in non-human primates. A combined measles-dengue vaccine might provide a one-shot approach to immunize children against both diseases where they co-exist.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med. 2004;10:S98–109. - PubMed
-
- Catteau A, Kalinina O, Wagner MC, Deubel V, Courageot MP, et al. Dengue virus M protein contains a proapoptotic sequence referred to as ApoptoM. J Gen Virol. 2003;84:2781–2793. - PubMed
-
- Halstead SB, O'Rourke EJ. Antibody-enhanced dengue virus infection in primate leukocytes. Nature. 1977;265:739–741. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

