Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse
- PMID: 18161012
- PMCID: PMC3372576
- DOI: 10.1007/s00213-007-1020-8
Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse
Abstract
Introduction: Individuals seeking treatment for their marijuana use rarely achieve sustained abstinence.
Objectives: The objectives of the study are to determine if THC, a cannabinoid agonist, and lofexidine, an alpha(2)-adrenergic receptor agonist, given alone and in combination, decreased symptoms of marijuana withdrawal and relapse, defined as a return to marijuana use after a period of abstinence.
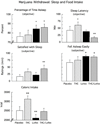
Materials and methods: Nontreatment-seeking, male volunteers (n = 8), averaging 12 marijuana cigarettes/day, were maintained on each of four medication conditions for 7 days: placebo, tetrahydrocannabinol (THC) (60 mg/day), lofexidine (2.4 mg/day), and THC (60 mg/day) combined with lofexidine (2.4 mg/day); each inpatient phase was separated by an outpatient washout phase. During the first three inpatient days, placebo marijuana was available for self-administration (withdrawal). For the next 4 days, active marijuana was available for self-administration (relapse). Participants paid for self-administered marijuana using study earnings. Self-administration, mood, task performance, food intake, and sleep were measured.
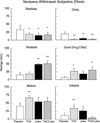
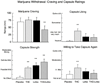
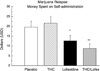
Results: THC reversed the anorexia and weight loss associated with marijuana withdrawal, and decreased a subset of withdrawal symptoms, but increased sleep onset latency, and did not decrease marijuana relapse. Lofexidine was sedating, worsened abstinence-related anorexia, and did not robustly attenuate withdrawal, but improved sleep and decreased marijuana relapse. The combination of lofexidine and THC produced the most robust improvements in sleep and decreased marijuana withdrawal, craving, and relapse in daily marijuana smokers relative to either medication alone.
Conclusions: These data suggest the combination of lofexidine and THC warrant further testing as a potential treatment for marijuana dependence.
Figures
References
-
- Ajilore O, Stickgold R, Rittenhouse CD, Hobson JA. Nightcap: laboratory and homebased evaluation of a portable sleep monitor. Psychophysiology. 1995;32:92–98. - PubMed
-
- Anggadiredja K, Yamaguchi T, Tanaka H, Shoyama Y, Watanabe S, Yamamoto T. Prostaglandin E2 attenuates SR141716A-precipitated withdrawal in tetrahydrocannabinol-dependent mice. Brain Res. 2003;966:47–53. - PubMed
-
- Anthony JC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances and inhalants: basic findings from the National Comorbidity Survey. Exp Clin Psychopharmacol. 1994;2:244–268.
-
- Balerio GN, Aso E, Berrendero F, Murtra P, Maldonado R. Delta9-tetrahydrocannabinol decreases somatic and motivational manifestations of nicotine withdrawal in mice. Eur J Neurosci. 2004;20:2737–2748. - PubMed
-
- Bearn J, Gossop M, Srang J. Accelerated lofexidine treatment regimen compared with conventional lofexidine and methadone treatment for inpatient opiate detoxification. Drug Alcohol Depend. 1998;50:227–232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

