Inhibition of Müller glial cell division blocks regeneration of the light-damaged zebrafish retina
- PMID: 18161852
- PMCID: PMC3711086
- DOI: 10.1002/dneu.20596
Inhibition of Müller glial cell division blocks regeneration of the light-damaged zebrafish retina
Abstract
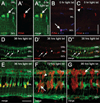
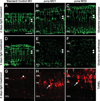
The adult zebrafish retina possesses a robust regenerative response. In the light-damaged retina, Müller glial cell divisions precede regeneration of rod and cone photoreceptors. Neuronal progenitors, which arise from the Müller glia, continue to divide and use the Müller glial cell processes to migrate to the outer nuclear layer and replace the lost photoreceptors. We tested the necessity of Müller glial cell division for photoreceptor regeneration. As knockdown tools were unavailable for use in the adult zebrafish retina, we developed a method to conditionally inhibit the expression of specific proteins by in vivo electroporation of morpholinos. We determined that two separate morpholinos targeted against the proliferating cell nuclear antigen (PCNA) mRNA reduced PCNA protein levels. Furthermore, injection and in vivo electroporation of PCNA morpholinos immediately prior to starting intense light exposure inhibited both Müller glial cell proliferation and neuronal progenitor marker Pax6 expression. PCNA knockdown additionally resulted in decreased expression of glutamine synthetase in Müller glia and Müller glial cell death, while amacrine and ganglion cells were unaffected. Finally, histological and immunological methods showed that long-term effects of PCNA knockdown resulted in decreased numbers of Müller glia and the failure to regenerate rod photoreceptors, short single cones, and long single cones. These data suggest that Müller glial cell division is necessary for proper photoreceptor regeneration in the light-damaged zebrafish retina and are consistent with the Müller glia serving as the source of neuronal progenitor cells in regenerating teleost retinas.
Figures





References
-
- Cameron DA, Gentile KL, Middleton FA, Yurco P. Gene expression profiles of intact and regenerating zebrafish retina. Mol Vis. 2005;11:775–791. - PubMed
-
- Choi HJ, Choi YH, Yee SB, Im E, Jung JH, Kim ND. Ircinin-1 induces cell cycle arrest and apoptosis in SK-MEL-2 human melanoma cells. Mol Carcinog. 2005;44:162–173. - PubMed
-
- Das AV, Mallya KB, Zhao X, Ahmad F, Bhattacharya S, Thoreson WB, Hegde GV, et al. Neural stem cell properties of Muller glia in the mammalian retina: Regulation by Notch and Wnt signaling. Dev Biol. 2006;299:283–302. - PubMed
-
- Detrich HW, Westerfield M, Zon LI. Methods in Cell Biology: The Zebrafish Biology. New York: Academic Press; 1999.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

