GATA3-driven Th2 responses inhibit TGF-beta1-induced FOXP3 expression and the formation of regulatory T cells
- PMID: 18162042
- PMCID: PMC2222968
- DOI: 10.1371/journal.pbio.0050329
GATA3-driven Th2 responses inhibit TGF-beta1-induced FOXP3 expression and the formation of regulatory T cells
Abstract
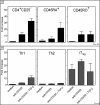
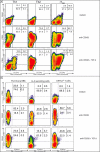
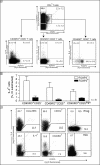
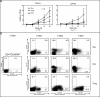
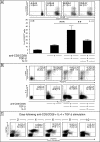
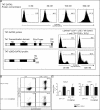
Transcription factors act in concert to induce lineage commitment towards Th1, Th2, or T regulatory (Treg) cells, and their counter-regulatory mechanisms were shown to be critical for polarization between Th1 and Th2 phenotypes. FOXP3 is an essential transcription factor for natural, thymus-derived (nTreg) and inducible Treg (iTreg) commitment; however, the mechanisms regulating its expression are as yet unknown. We describe a mechanism controlling iTreg polarization, which is overruled by the Th2 differentiation pathway. We demonstrated that interleukin 4 (IL-4) present at the time of T cell priming inhibits FOXP3. This inhibitory mechanism was also confirmed in Th2 cells and in T cells of transgenic mice overexpressing GATA-3 in T cells, which are shown to be deficient in transforming growth factor (TGF)-beta-mediated FOXP3 induction. This inhibition is mediated by direct binding of GATA3 to the FOXP3 promoter, which represses its transactivation process. Therefore, this study provides a new understanding of tolerance development, controlled by a type 2 immune response. IL-4 treatment in mice reduces iTreg cell frequency, highlighting that therapeutic approaches that target IL-4 or GATA3 might provide new preventive strategies facilitating tolerance induction particularly in Th2-mediated diseases, such as allergy.
Conflict of interest statement
Figures


References
-
- Kurata H, Lee HJ, O'Garra A, Arai N. Ectopic expression of activated Stat6 induces the expression of Th2-specific cytokines and transcription factors in developing Th1 cells. Immunity. 1999;11:677–688. - PubMed
-
- Ouyang W, Ranganath SH, Weindel K, Bhattacharya D, Murphy TL, et al. Inhibition of Th1 development mediated by GATA-3 through an IL-4-independent mechanism. Immunity. 1998;9:745–755. - PubMed
-
- Zhu J, Yamane H, Cote-Sierra J, Guo L, Paul WE. GATA-3 promotes Th2 responses through three different mechanisms: induction of Th2 cytokine production, selective growth of Th2 cells and inhibition of Th1 cell-specific factors. Cell Res. 2006;16:3–10. - PubMed
-
- Hwang ES, Szabo SJ, Schwartzberg PL, Glimcher LH. T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science. 2005;307:430–433. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

