Withdrawal of inhaled corticosteroids in people with COPD in primary care: a randomised controlled trial
- PMID: 18162137
- PMCID: PMC2245934
- DOI: 10.1186/1465-9921-8-93
Withdrawal of inhaled corticosteroids in people with COPD in primary care: a randomised controlled trial
Abstract
Background: Guidelines recommend inhaled corticosteroids (ICS) for patients with severe chronic obstructive pulmonary disease (COPD). Most COPD patients are managed in primary care and receive ICS long-term and irrespective of severity. The effect of withdrawing ICS from COPD patients in primary care is unknown.
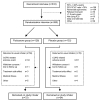
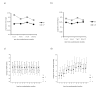
Methods: In a pragmatic randomised, double-blind, placebo-controlled trial in 31 practices, 260 COPD patients stopped their usual ICS (median duration of use 8 years) and were allocated to 500 mcg fluticasone propionate twice daily (n = 128), or placebo (n = 132). Follow-up assessments took place at three monthly intervals for a year at the patients' practice. Our primary outcome was COPD exacerbation frequency. Secondary outcomes were time to first COPD exacerbation, reported symptoms, peak expiratory flow rate and reliever inhaler use, and lung function and health related quality of life.
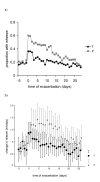
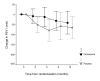
Results: In patients randomised to placebo, COPD exacerbation risk over one year was RR: 1.11 (CI: 0.91-1.36). Patients taking placebo were more likely to return to their usual ICS following exacerbation, placebo: 61/128 (48%); fluticasone: 34/132 (26%), OR: 2.35 (CI: 1.38-4.05). Exacerbation risk whilst taking randomised treatment was significantly raised in the placebo group 1.48 (CI: 1.17-1.86). Patients taking placebo exacerbated earlier (median time to first exacerbation: placebo (days): 44 (CI: 29-59); fluticasone: 63 (CI: 53-74), log rank 3.81, P = 0.05) and reported increased wheeze. In a post-hoc analysis, patients with mild COPD taking placebo had increased exacerbation risk RR: 1.94 (CI: 1.20-3.14).
Conclusion: Withdrawal of long-term ICS in COPD patients in primary care increases risk of exacerbation shortens time to exacerbation and causes symptom deterioration. Patients with mild COPD may be at increased risk of exacerbation after withdrawal.
Trial registration: ClinicalTrials.gov NCT00440687.
Figures

References
-
- Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1418–1422. - PubMed
-
- Miravitlles M, Ferrer M, Pont A, Zalacain R, Alvarez-Sala JL, Masa F, Verea H, Murio C, Ros F, Vidal R. Effect of exacerbations on quality of life in patients with chronic obstructive pulmonary disease: a 2 year follow up study. Thorax. 2004;59(5):387–395. doi: 10.1136/thx.2003.008730. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

