Insulin mutation screening in 1,044 patients with diabetes: mutations in the INS gene are a common cause of neonatal diabetes but a rare cause of diabetes diagnosed in childhood or adulthood
- PMID: 18162506
- PMCID: PMC7611804
- DOI: 10.2337/db07-1405
Insulin mutation screening in 1,044 patients with diabetes: mutations in the INS gene are a common cause of neonatal diabetes but a rare cause of diabetes diagnosed in childhood or adulthood
Abstract
Objective: Insulin gene (INS) mutations have recently been described as a cause of permanent neonatal diabetes (PND). We aimed to determine the prevalence, genetics, and clinical phenotype of INS mutations in large cohorts of patients with neonatal diabetes and permanent diabetes diagnosed in infancy, childhood, or adulthood.
Research design and methods: The INS gene was sequenced in 285 patients with diabetes diagnosed before 2 years of age, 296 probands with maturity-onset diabetes of the young (MODY), and 463 patients with young-onset type 2 diabetes (nonobese, diagnosed <45 years). None had a molecular genetic diagnosis of monogenic diabetes.
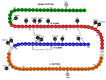
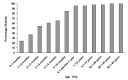
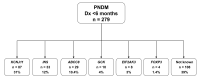
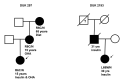
Results: We identified heterozygous INS mutations in 33 of 141 probands diagnosed at <6 months, 2 of 86 between 6 and 12 months, and none of 58 between 12 and 24 months of age. Three known mutations (A24D, F48C, and R89C) account for 46% of cases. There were six novel mutations: H29D, L35P, G84R, C96S, S101C, and Y103C. INS mutation carriers were all insulin treated from diagnosis and were diagnosed later than ATP-sensitive K(+) channel mutation carriers (11 vs. 8 weeks, P < 0.01). In 279 patients with PND, the frequency of KCNJ11, ABCC8, and INS gene mutations was 31, 10, and 12%, respectively. A heterozygous R6C mutation cosegregated with diabetes in a MODY family and is probably pathogenic, but the L68M substitution identified in a patient with young-onset type 2 diabetes may be a rare nonfunctional variant.
Conclusions: We conclude that INS mutations are the second most common cause of PND and a rare cause of MODY. Insulin gene mutation screening is recommended for all diabetic patients diagnosed before 1 year of age.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Comment in
-
Insulin mutations in diabetes: the clinical spectrum.Diabetes. 2008 Apr;57(4):799-800. doi: 10.2337/db08-0116. Diabetes. 2008. PMID: 18375443 No abstract available.
-
Comment on: Edghill et al. (2008) Insulin mutation screening in 1,044 patients with diabetes: mutations in the INS gene are a common cause of neonatal diabetes but a rare cause of diabetes diagnosed in childhood or adulthood: Diabetes 57:1034-1042, 2008.Diabetes. 2008 May;57(5):e9. doi: 10.2337/db08-0091. Diabetes. 2008. PMID: 18443369 No abstract available.
References
-
- Iafusco D, Stazi MA, Cotichini R, Cotellessa M, Martinucci ME, Mazzella M, Cherubini V, Barbetti F, Martinetti M, Cerutti F, Prisco F. Permanent diabetes mellitus in the first year of life. Diabetologia. 2002;45:798–804. - PubMed
-
- Edghill EL, Dix RJ, Flanagan SE, Bingley PJ, Hattersley AT, Ellard S, Gillespie KM. HLA genotyping supports a nonautoimmune etiology in patients diagnosed with diabetes under the age of 6 months. Diabetes. 2006;55:1895–1898. - PubMed
-
- Stanik J, Gasperikova D, Paskova M, Barak L, Javorkova J, Jancova E, Ciljakova M, Hlava P, Michalek J, Flanagan SE, Pearson E, et al. Prevalence of permanent neonatal diabetes in Slovakia and successful replacement of insulin with sulfonylurea therapy in KCNJ11 and ABCC8 mutation carriers. J Clin Endocrinol Metab. 2007;92:1276–1282. - PMC - PubMed
-
- Polak M, Shield J. Neonatal and very-early-onset diabetes mellitus. Semin Neonatol. 2004;9:59–65. - PubMed
-
- Gloyn AL, Pearson ER, Antcliff JF, Proks P, Bruining GJ, Slingerland AS, Howard N, Srinivasan S, Silva JM, Molnes J, Edghill EL, et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med. 2004;350:1838–1849. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 067463/WT_/Wellcome Trust/United Kingdom
- R29 DK044752/DK/NIDDK NIH HHS/United States
- DK-73541/DK/NIDDK NIH HHS/United States
- DK-20595/DK/NIDDK NIH HHS/United States
- R01 DK077489/DK/NIDDK NIH HHS/United States
- DK-44752/DK/NIDDK NIH HHS/United States
- DK-77489/DK/NIDDK NIH HHS/United States
- P30 DK020595/DK/NIDDK NIH HHS/United States
- P60 DK020595/DK/NIDDK NIH HHS/United States
- DK-13914/DK/NIDDK NIH HHS/United States
- R01 DK044752/DK/NIDDK NIH HHS/United States
- R01 DK073541/DK/NIDDK NIH HHS/United States
- R01 DK013914/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

