Activity-dependent phosphorylation of Ser187 is required for SNAP-25-negative modulation of neuronal voltage-gated calcium channels
- PMID: 18162553
- PMCID: PMC2224210
- DOI: 10.1073/pnas.0706211105
Activity-dependent phosphorylation of Ser187 is required for SNAP-25-negative modulation of neuronal voltage-gated calcium channels
Abstract
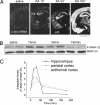
Synaptosomal-associated protein of 25 kDa (SNAP-25) is a SNARE protein that regulates neurotransmission by the formation of a complex with syntaxin 1 and synaptobrevin/VAMP2. SNAP-25 also reduces neuronal calcium responses to stimuli, but neither the functional relevance nor the molecular mechanisms of this modulation have been clarified. In this study, we demonstrate that hippocampal slices from Snap25(+/-) mice display a significantly larger facilitation and that higher calcium peaks are reached after depolarization by Snap25(-/-) and Snap25(+/-) cultured neurons compared with wild type. We also show that SNAP-25b modulates calcium dynamics by inhibiting voltage-gated calcium channels (VGCCs) and that PKC phosphorylation of SNAP-25 at ser187 is essential for this process, as indicated by the use of phosphomimetic (S187E) or nonphosphorylated (S187A) mutants. Neuronal activity is the trigger that induces the transient phosphorylation of SNAP-25 at ser187. Indeed, enhancement of network activity increases the levels of phosphorylated SNAP-25, whereas network inhibition reduces the extent of protein phosphorylation. A transient peak of SNAP-25 phosphorylation also is detectable in rat hippocampus in vivo after i.p. injection with kainate to induce seizures. These findings demonstrate that differences in the expression levels of SNAP-25 impact on calcium dynamics and neuronal plasticity, and that SNAP-25 phosphorylation, by promoting inhibition of VGCCs, may mediate a negative feedback modulation of neuronal activity during intense activation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

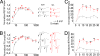


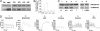
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

