Isc1p plays a key role in hydrogen peroxide resistance and chronological lifespan through modulation of iron levels and apoptosis
- PMID: 18162582
- PMCID: PMC2262964
- DOI: 10.1091/mbc.e07-06-0604
Isc1p plays a key role in hydrogen peroxide resistance and chronological lifespan through modulation of iron levels and apoptosis
Abstract
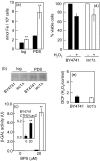
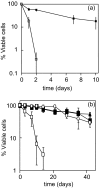
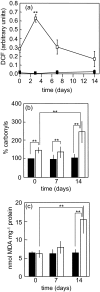
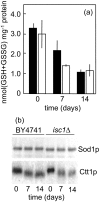
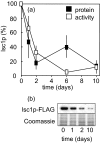
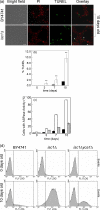
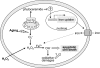
The inositolphosphosphingolipid phospholipase C (Isc1p) of Saccharomyces cerevisiae belongs to the family of neutral sphingomyelinases that generates the bioactive sphingolipid ceramide. In this work the role of Isc1p in oxidative stress resistance and chronological lifespan was investigated. Loss of Isc1p resulted in a higher sensitivity to hydrogen peroxide that was associated with an increase in oxidative stress markers, namely intracellular oxidation, protein carbonylation, and lipid peroxidation. Microarray analysis showed that Isc1p deficiency up-regulated the iron regulon leading to increased levels of iron, which is known to catalyze the production of the highly reactive hydroxyl radicals via the Fenton reaction. In agreement, iron chelation suppressed hydrogen peroxide sensitivity of isc1Delta mutants. Cells lacking Isc1p also displayed a shortened chronological lifespan associated with oxidative stress markers and aging of parental cells was correlated with a decrease in Isc1p activity. The analysis of DNA fragmentation and caspase-like activity showed that Isc1p deficiency increased apoptotic cell death associated with oxidative stress and aging. Furthermore, deletion of Yca1p metacaspase suppressed the oxidative stress sensitivity and premature aging phenotypes of isc1Delta mutants. These results indicate that Isc1p plays an important role in the regulation of cellular redox homeostasis, through modulation of iron levels, and of apoptosis.
Figures




References
-
- Aerts A. M., Francois I. E., Bammens L., Cammue B. P., Smets B., Winderickx J., Accardo S., De Vos D. E., Thevissen K. Level of M(IP)2C sphingolipid affects plant defensin sensitivity, oxidative stress resistance and chronological life-span in yeast. FEBS Lett. 2006;580:1903–1907. - PubMed
-
- Almeida B., Sampaio-Marques B., Carvalho J., Silva M. T., Leao C., Rodrigues F., Ludovico P. An atypical active cell death process underlies the fungicidal activity of ciclopirox olamine against the yeast Saccharomyces cerevisiae. FEMS Yeast Res. 2007;7:404–412. - PubMed
-
- Ausubel F. A., Brent R., Kingston D., Moore D., Seidman J. G., Smith J. A., Struhl K. Current Protocols in Molecular Biology. New York: John Wiley and Sons; 1998.
-
- Belinha I., Amorim M. A., Rodrigues P., de Freitas V., Moradas-Ferreira P., Mateus N., Costa V. Quercetin increases oxidative stress resistance and longevity in Saccharomyces cerevisiae. J. Agric. Food Chem. 2007;55:2446–2451. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

