Caveolin-1 and -2 interact with connexin43 and regulate gap junctional intercellular communication in keratinocytes
- PMID: 18162583
- PMCID: PMC2262971
- DOI: 10.1091/mbc.e07-06-0596
Caveolin-1 and -2 interact with connexin43 and regulate gap junctional intercellular communication in keratinocytes
Abstract
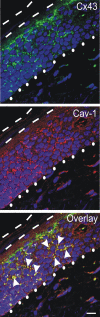
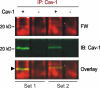
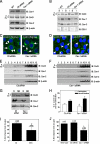
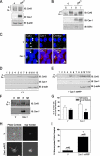
Connexin43 (Cx43) has been reported to interact with caveolin (Cav)-1, but the role of this association and whether other members of the caveolin family bind Cx43 had yet to be established. In this study, we show that Cx43 coimmunoprecipitates and colocalizes with Cav-1 and Cav-2 in rat epidermal keratinocytes. The colocalization of Cx43 with Cav-1 was confirmed in keratinocytes from human epidermis in vivo. Our mutation and Far Western analyses revealed that the C-terminal tail of Cx43 is required for its association with Cavs and that the Cx43/Cav-1 interaction is direct. Our results indicate that newly synthesized Cx43 interacts with Cavs in the Golgi apparatus and that the Cx43/Cavs complex also exists at the plasma membrane in lipid rafts. Using overexpression and small interfering RNA approaches, we demonstrated that caveolins regulate gap junctional intercellular communication (GJIC) and that the presence of Cx43 in lipid raft domains may contribute to the mechanism modulating GJIC. Our results suggest that the Cx43/Cavs association occurs during exocytic transport, and they clearly indicate that caveolin regulates GJIC.
Figures




Similar articles
-
The tumor-suppressive function of Connexin43 in keratinocytes is mediated in part via interaction with caveolin-1.Cancer Res. 2010 May 15;70(10):4222-32. doi: 10.1158/0008-5472.CAN-09-3281. Epub 2010 Apr 20. Cancer Res. 2010. PMID: 20406988
-
Down-regulation of Connexin43 expression reveals the involvement of caveolin-1 containing lipid rafts in human U251 glioblastoma cell invasion.Mol Carcinog. 2012 Nov;51(11):845-60. doi: 10.1002/mc.20853. Epub 2011 Aug 31. Mol Carcinog. 2012. PMID: 21882259
-
Connexin family members target to lipid raft domains and interact with caveolin-1.Biochemistry. 2002 May 7;41(18):5754-64. doi: 10.1021/bi0121656. Biochemistry. 2002. PMID: 11980479
-
The connexin 43 C-terminus: A tail of many tales.Biochim Biophys Acta Biomembr. 2018 Jan;1860(1):48-64. doi: 10.1016/j.bbamem.2017.05.008. Epub 2017 May 16. Biochim Biophys Acta Biomembr. 2018. PMID: 28526583 Review.
-
Phosphorylation of connexin43 induced by Src: regulation of gap junctional communication between transformed cells.Exp Cell Res. 2007 Dec 10;313(20):4083-90. doi: 10.1016/j.yexcr.2007.09.010. Epub 2007 Sep 20. Exp Cell Res. 2007. PMID: 17956757 Review.
Cited by
-
Gap Junction-Dependent and -Independent Functions of Connexin43 in Biology.Biology (Basel). 2022 Feb 11;11(2):283. doi: 10.3390/biology11020283. Biology (Basel). 2022. PMID: 35205149 Free PMC article. Review.
-
Caveolin-1 as a pathophysiological factor and target in psoriasis.NPJ Aging Mech Dis. 2019 Feb 5;5:4. doi: 10.1038/s41514-019-0034-x. eCollection 2019. NPJ Aging Mech Dis. 2019. PMID: 30729030 Free PMC article. Review.
-
Cx50 requires an intact PDZ-binding motif and ZO-1 for the formation of functional intercellular channels.Mol Biol Cell. 2011 Dec;22(23):4503-12. doi: 10.1091/mbc.E11-05-0438. Epub 2011 Sep 30. Mol Biol Cell. 2011. PMID: 21965293 Free PMC article.
-
Regulation of cellular communication by signaling microdomains in the blood vessel wall.Pharmacol Rev. 2014 Mar 26;66(2):513-69. doi: 10.1124/pr.112.007351. Print 2014. Pharmacol Rev. 2014. PMID: 24671377 Free PMC article. Review.
-
Role of membrane microdomains in cardiac protection: strategies for diabetic cardiomyopathy.Am J Physiol Heart Circ Physiol. 2025 Aug 1;329(2):H572-H591. doi: 10.1152/ajpheart.00139.2025. Epub 2025 Jul 10. Am J Physiol Heart Circ Physiol. 2025. PMID: 40637400 Free PMC article. Review.
References
-
- Barbuti A., Gravante B., Riolfo M., Milanesi R., Terragni B., DiFrancesco D. Localization of pacemaker channels in lipid rafts regulates channel kinetics. Circ. Res. 2004;94:1325–1331. - PubMed
-
- Barth K., Gentsch M., Blasche R., Pfuller A., Parshyna I., Koslowski R., Barth G., Kasper M. Distribution of caveolin-1 and connexin43 in normal and injured alveolar epithelial R3/1 cells. Histochem. Cell Biol. 2005;123:239–247. - PubMed
-
- Brainard A. M., Miller A. J., Martens J. R., England S. K. Maxi-K channels localize to caveolae in human myometrium: a role for an actin-channel-caveolin complex in the regulation of myometrial smooth muscle K+ current. Am. J. Physiol. 2005;289:C49–C57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

