Specificity of RCN1-mediated protein phosphatase 2A regulation in meristem organization and stress response in roots
- PMID: 18162590
- PMCID: PMC2245836
- DOI: 10.1104/pp.107.112995
Specificity of RCN1-mediated protein phosphatase 2A regulation in meristem organization and stress response in roots
Abstract
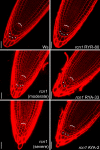
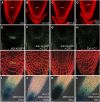
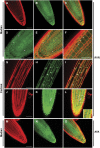
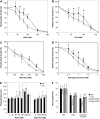
Protein dephosphorylation by the serine/threonine protein phosphatase 2A (PP2A) modulates a broad array of cellular functions. PP2A normally acts as a heterotrimeric holoenzyme complex comprising a catalytic subunit bound by regulatory A and B subunits. Characterization of the regulatory A subunit isoforms (ROOTS CURL IN NAPHTHYLPHTHALAMIC ACID1 [RCN1], PP2AA2, and PP2AA3) of Arabidopsis thaliana PP2A has shown that RCN1 plays a primary role in controlling root and hypocotyl PP2A activity in seedlings. Here we show that hypocotyl and root growth exhibit different requirements for RCN1-mediated regulation of PP2A activity. Roots of rcn1 mutant seedlings exhibit characteristic abnormalities in cell division patterns at the root apical meristem, as well as reduced growth under ionic, osmotic, and oxidative stress conditions. We constructed chimeric A subunit genes and found that restoration of normal root tip development in rcn1 plants requires both regulatory and coding sequences of RCN1, whereas the hypocotyl elongation defect of rcn1 plants can be complemented by either RCN1 or PP2AA3 transgenes. Furthermore, the RCN1 and PP2AA3 proteins exhibit ubiquitous subcellular localization patterns in seedlings and both associate with membrane compartments. Together, these results show that RCN1-containing PP2A has unique functions that cannot be attributed to isoform-specific expression and localization patterns. Postembryonic RCN1 function is required to maintain normal auxin distribution and stem cell function at the root apex. Our data show that RCN1-regulated phosphatase activity plays a unique role in regulating postembryonic root development and stress response.
Figures



References
-
- Ammerer G (1983) Expression of genes in yeast using the ADC1 promoter. Methods Enzymol 101 192–201 - PubMed
-
- Andrade MA, Bork P (1995) HEAT repeats in the Huntington's disease protein. Nat Genet 11 115–116 - PubMed
-
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (1992) Current Protocols in Molecular Biology. Wiley-Interscience, New York
-
- Bechtold N, Ellis H, Pelletier G (1993) In planta Agrobacterium-mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C R Acad Sci Ser III Sci Vie 316 1194–1199
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

