Silicon uptake in diatoms revisited: a model for saturable and nonsaturable uptake kinetics and the role of silicon transporters
- PMID: 18162598
- PMCID: PMC2259041
- DOI: 10.1104/pp.107.107094
Silicon uptake in diatoms revisited: a model for saturable and nonsaturable uptake kinetics and the role of silicon transporters
Abstract
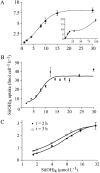
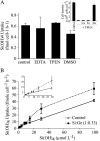
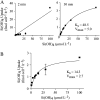
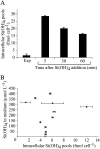
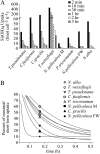
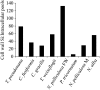
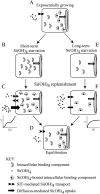
The silicic acid uptake kinetics of diatoms were studied to provide a mechanistic explanation for previous work demonstrating both nonsaturable and Michaelis-Menten-type saturable uptake. Using (68)Ge(OH)(4) as a radiotracer for Si(OH)(4), we showed a time-dependent transition from nonsaturable to saturable uptake kinetics in multiple diatom species. In cells grown under silicon (Si)-replete conditions, Si(OH)(4) uptake was initially nonsaturable but became saturable over time. Cells prestarved for Si for 24 h exhibited immediate saturable kinetics. Data suggest nonsaturability was due to surge uptake when intracellular Si pool capacity was high, and saturability occurred when equilibrium was achieved between pool capacity and cell wall silica incorporation. In Thalassiosira pseudonana at low Si(OH)(4) concentrations, uptake followed sigmoidal kinetics, indicating regulation by an allosteric mechanism. Competition of Si(OH)(4) uptake with Ge(OH)(4) suggested uptake at low Si(OH)(4) concentrations was mediated by Si transporters. At high Si(OH)(4), competition experiments and nonsaturability indicated uptake was not carrier mediated and occurred by diffusion. Zinc did not appear to be directly involved in Si(OH)(4) uptake, in contrast to a previous suggestion. A model for Si(OH)(4) uptake in diatoms is presented that proposes two control mechanisms: active transport by Si transporters at low Si(OH)(4) and diffusional transport controlled by the capacity of intracellular pools in relation to cell wall silica incorporation at high Si(OH)(4). The model integrates kinetic and equilibrium components of diatom Si(OH)(4) uptake and consistently explains results in this and previous investigations.
Figures
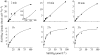
References
-
- Azam F (1974) Silicic acid uptake in diatoms studied with [68Ge]germanic acid as a tracer. Planta 121 205–212 - PubMed
-
- Azam F, Hemmsingsen BB, Volcani BE (1974) Role of silicon in diatom metabolism. V. Silicic acid transport and metabolism in the heterotrophic diatom Nitzschia alba. Arch Microbiol 97 103–114 - PubMed
-
- Azam F, Volcani B (1974) Role of silicon in diatom metabolism. VI. Active transport of germanic acid in the heterotrophic diatom Nitzschia alba. Arch Microbiol 101 1–8 - PubMed
-
- Bhattacharyya P, Volcani BE (1983) Isolation of silicate ionophores from the apochlorotic diatom Nitzschia alba. Biochem Biophys Res Commun 114 365–372 - PubMed
-
- Binder BJ, Chisholm SW (1980) Changes in the soluble silicon pool size in the marine diatom Thalassiosira fluviatilis. Marine Biology Letters 1 205–212
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

