The catalytic asymmetric total synthesis of elatol
- PMID: 18163634
- PMCID: PMC2533138
- DOI: 10.1021/ja710294k
The catalytic asymmetric total synthesis of elatol
Abstract
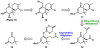
Described in this report is the first total synthesis of elatol, a halogenated sesquiterpene in the chamigrene natural product family. The key disconnections in our synthetic approach include an enantioselective decarboxylative allylation to form the all-carbon quaternary stereocenter and a ring-closing olefin metathesis to concomitantly form the spirocyclic core as well as the fully substituted chlorinated olefin. This strategy represents a general platform for accessing the chamigrene natural product family, as demonstrated by the synthesis of (+)-laurencenone B as an intermediate in our route.
Figures
Similar articles
-
A general enantioselective route to the chamigrene natural product family.Tetrahedron. 2010 Mar 26;66(26):4668-4686. doi: 10.1016/j.tet.2010.04.128. Tetrahedron. 2010. PMID: 20798895 Free PMC article.
-
Enhancement of enantioselectivity by THF in asymmetric mo-catalyzed olefin metathesis. Catalytic enantioselective synthesis of cyclic tertiary ethers and spirocycles.J Am Chem Soc. 2002 Sep 11;124(36):10779-84. doi: 10.1021/ja020671s. J Am Chem Soc. 2002. PMID: 12207534
-
Mo-catalyzed asymmetric olefin metathesis in target-oriented synthesis: enantioselective synthesis of (+)-africanol.Proc Natl Acad Sci U S A. 2004 Apr 20;101(16):5805-9. doi: 10.1073/pnas.0307589101. Epub 2004 Mar 31. Proc Natl Acad Sci U S A. 2004. PMID: 15056762 Free PMC article.
-
Enantioselective synthesis of substituted oxindoles and spirooxindoles with applications in drug discovery.Curr Opin Drug Discov Devel. 2010;13(6):758-76. Curr Opin Drug Discov Devel. 2010. PMID: 21061236 Review.
-
Enantioselective formation of quaternary carbon stereocenters in natural product synthesis: a recent update.Nat Prod Rep. 2020 Feb 26;37(2):276-292. doi: 10.1039/c9np00039a. Nat Prod Rep. 2020. PMID: 31515549 Review.
Cited by
-
Design, Synthesis, and Antifouling Activity of Glucosamine-Based Isocyanides.Mar Drugs. 2017 Jun 29;15(7):203. doi: 10.3390/md15070203. Mar Drugs. 2017. PMID: 28661419 Free PMC article.
-
Enantioselective total synthesis of (+)-cassiol.Org Lett. 2009 Jan 15;11(2):293-5. doi: 10.1021/ol802410t. Org Lett. 2009. PMID: 19093836 Free PMC article.
-
Transcriptomic analysis of the red seaweed Laurencia dendroidea (Florideophyceae, Rhodophyta) and its microbiome.BMC Genomics. 2012 Sep 17;13:487. doi: 10.1186/1471-2164-13-487. BMC Genomics. 2012. PMID: 22985125 Free PMC article.
-
Enantioselective total synthesis of the reported structures of (-)-9-epi-presilphiperfolan-1-ol and (-)-presilphiperfolan-1-ol: structural confirmation and reassignment and biosynthetic insights.Angew Chem Int Ed Engl. 2012 Sep 17;51(38):9674-8. doi: 10.1002/anie.201205276. Epub 2012 Aug 22. Angew Chem Int Ed Engl. 2012. PMID: 22915502 Free PMC article. No abstract available.
-
Palladium-Catalyzed Asymmetric Alkylation in the Synthesis of Cyclopentanoid and Cycloheptanoid Core Structures Bearing All-Carbon Quaternary Stereocenters.Tetrahedron. 2011 Dec 30;67(52):10234-10248. doi: 10.1016/j.tet.2011.10.031. Tetrahedron. 2011. PMID: 22347731 Free PMC article.
References
-
- Erickson KL. Constituents of Laurencia. In: Scheuer PJ, editor. Marine Natural Products: Chemical and Biological Perspectives. V. Academic Press; New York: 1983. pp. 131–257.
- Dorta A, Díaz-Marrero AR, Cueto M, D’Croz L, Maté JL, Darias J. Tetrahedron Lett. 2004;45:7065–7068.
-
-
Originally isolated from the marine alga Laurencia elata: Sims JJ, Lin GHY, Wing RM. Tetrahedron Lett. 1974;15:3487–3490.
-
-
-
Granado I, Caballero P. Sci Mar. 1995;59(Supl 1):31–39.de Nys R, Leya T, Maximilien R, Afsar A, Nair PSR, Steinberg PD. Biofouling. 1996;10:213–224.Martín JD, Pérez C, Ravelo JL. J Am Chem Soc. 1986;108:7801–7811.Vairappan CS, Daitoh M, Suzuki M, Abe T, Masuda M. Phytochemistry. 2001;58:291–297.Vairappan CS. Biomol Eng. 2003;20:255–259.König GM, Wright AD. J Nat Prod. 1997;60:967–970.(g) HeLa: IC50 = 4.1 μM (lag phase), 1.3 μM (log phase); Hep-2: IC50 = 2.4 μM (lag phase), 2.0 μM (log phase); Dias T, Brito I, Moujir L, Paiz N, Darias J, Cueto M. J Nat Prod. 2005;68:1677–1679.
-
-
-
For the preparation of elatol (1) via the degradation of isoobtusol, see: González AG, Darias J, Díaz A, Fourneron JD, Martín JD, Pérez C. Tetrahedron Lett. 1976;17:3051–3054.González AG, Martín JD, Martín VS, Martínez-Ripoll M, Fayos J. Tetrahedron Lett. 1979;20:2717–2718.González AG, Martín JD, Martín VS, Norte M, Pérez R. Tetrahedron Lett. 1982;23:2395–2398.
-
-
-
For lead references on the total syntheses of other chamigrene natural products, see: Taber DF, Sikkander MI, Storck PH. J Org Chem. 2007;72:4098–4101.Srikrishna A, Lakshmi BV, Mathews M. Tetrahedron Lett. 2006;47:2103–2106.Chen YJ, Wang CY, Lin WY. Tetrahedron. 1996;52:13181–13188.Hatsui T, Wang JJ, Takeshita H. Bull Chem Soc Jpn. 1994;67:2507–2513. Also see ref 3c.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources