BGLAP is expressed in pancreatic cancer cells and increases their growth and invasion
- PMID: 18163903
- PMCID: PMC2245975
- DOI: 10.1186/1476-4598-6-83
BGLAP is expressed in pancreatic cancer cells and increases their growth and invasion
Abstract
Background: Bone gamma-carboxyglutamate protein (BGLAP; osteocalcin) is a small, highly conserved molecule first identified in the mineralized matrix of bone. It has been implicated in the pathophysiology of various malignancies. In this study, we analyzed the expression and role of BGLAP in the normal human pancreas, chronic pancreatitis (CP), and pancreatic ductal adenocarcinoma (PDAC) using quantitative RT-PCR, immunohistochemistry, immunocytochemistry and enzyme immunoassays, as well as cell proliferation and invasion assays. Gene silencing was carried out using specific siRNA molecules.
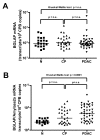
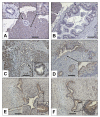
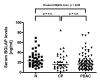
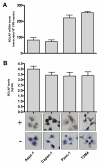
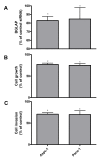
Results: Compared to the normal pancreas, BGLAP mRNA and protein levels were not significantly different in CP and PDAC tissues. BGLAP was faintly present in the cytoplasm of normal acinar cells but was strongly expressed in the cytoplasm and nuclei of tubular complexes and PanIN lesions of CP and PDAC tissues. Furthermore, BGLAP expression was found in the cancer cells in PDAC tissues as well as in 4 cultured pancreatic cancer cell lines. TNFalpha reduced BGLAP mRNA and protein expression levels in pancreatic cancer cell lines. In addition, BGLAP silencing led to reduction of both cell growth and invasion in those cells.
Conclusion: BGLAP is expressed in pancreatic cancer cells, where it potentially increases pancreatic cancer cell growth and invasion through autocrine and/or paracrine mechanisms.
Figures


References
-
- Hauschka PV. Osteocalcin: the vitamin K-dependent Ca2+-binding protein of bone matrix. Haemostasis. 1986;16:258–272. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

