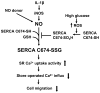
High glucose oxidizes SERCA cysteine-674 and prevents inhibition by nitric oxide of smooth muscle cell migration
- PMID: 18164028
- PMCID: PMC2394666
- DOI: 10.1016/j.yjmcc.2007.10.022
High glucose oxidizes SERCA cysteine-674 and prevents inhibition by nitric oxide of smooth muscle cell migration
Abstract
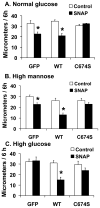
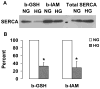
Nitric oxide (NO) causes S-glutathiolation of the reactive cysteine-674 in the sarcoplasmic/endoplasmic reticulum Ca(2+) ATPase (SERCA), thus increasing SERCA activity, and inhibiting Ca(2+) influx and migration of vascular smooth muscle cells (VSMC). Because increased VSMC migration contributes to accelerated neointimal growth and atherosclerosis in diabetes, the effect of culture of VSMC in high glucose (HG) was determined. Rat aortic VSMC were exposed to normal (5.5 mmol/L) or high (25 mmol/L) glucose for 3 days, and serum-induced cell migration during 6 h into a wounded cell monolayer was measured 5 min after adding the NO donor S-nitroso-N-acetylpenicillamine (SNAP) or 24 h after interleukin-1beta (IL-1beta) to express inducible nitric oxide synthase (iNOS). In normal glucose, SNAP or IL-1beta significantly inhibited migration in cells infected with adenovirus to express GFP or SERCA wild type (WT), but not with a C674S SERCA mutant. After HG, NO failed to inhibit migration, nor did it decrease calcium-dependent association of calmodulin with calcineurin, indicating that NO failed to decrease intracellular calcium levels via SERCA. In contrast, overexpression of SERCA WT, but not the SERCA C674S mutant, preserved the ability for NO to inhibit migration despite exposing the cells to HG. The antioxidant, Tempol, or overexpression of superoxide dismutase also prevented the effects of HG. Further studies showed that both biotinylated-iodoacetamide and NO-induced biotinylated glutathione labeling of SERCA C674 were decreased by HG, and a sequence-specific sulfonic acid antibody detected oxidation of the C674 SERCA thiol. These results indicate that failure of NO to inhibit migration in VSMC exposed to HG is due to oxidation of the SERCA reactive cysteine-674.
Figures






References
-
- Yasunari K, Kohno M, Kano H, Yokokawa K, Minami M, Yoshikawa J. Mechanisms of action of troglitazone in the prevention of high glucose-induced migration and proliferation of cultured coronary smooth muscle cells. Circ Res. 1997;81:953–62. - PubMed
-
- Yasunari K, Kohno M, Kano H, Yokokawa K, Minami M, Yoshikawa J. Antioxidants improve impaired insulin-mediated glucose uptake and prevent migration and proliferation of cultured rabbit coronary smooth muscle cells induced by high glucose. Circ. 1999;99(10):1370–8. - PubMed
-
- Scherberich A, Campos-Toimil M, Ronde P, Takeda K, Beretz A. Migration of human vascular smooth muscle cells involves serum-dependent repeated cytosolic calcium transients. J Cell Sci. 2000;113:653–62. - PubMed
-
- Cohen RA, Weisbrod RM, Gericke M, Yaghoubi M, Bierl C, Bolotina VM. Mechanism of nitric oxide-induced vasodilatation. Refilling of intracellular stores by sarcoplasmic reticulum Ca2+ ATPase and inhibition of store-operated Ca2+ influx. Circ Res. 1999;84:210–9. - PubMed
-
- Touyz RM. Reactive oxygen species as mediators of calcium signaling by angiotensin II: implications in vascular physiology and pathophysiology. Antioxid Redox Signal. 2005;7(9–10):1302–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

