Small-diameter biodegradable scaffolds for functional vascular tissue engineering in the mouse model
- PMID: 18164056
- PMCID: PMC2375856
- DOI: 10.1016/j.biomaterials.2007.11.041
Small-diameter biodegradable scaffolds for functional vascular tissue engineering in the mouse model
Abstract
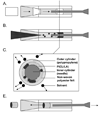
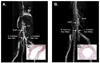
The development of neotissue in tissue engineered vascular grafts remains poorly understood. Advances in mouse genetic models have been highly informative in the study of vascular biology, but have been inaccessible to vascular tissue engineers due to technical limitations on the use of mouse recipients. To this end, we have developed a method for constructing sub-1mm internal diameter (ID) biodegradable scaffolds utilizing a dual cylinder chamber molding system and a hybrid polyester sealant scaled for use in a mouse model. Scaffolds constructed from either polyglycolic acid or poly-l-lactic acid nonwoven felts demonstrated sufficient porosity, biomechanical profile, and biocompatibility to function as vascular grafts. The scaffolds implanted as either inferior vena cava or aortic interposition grafts in SCID/bg mice demonstrated excellent patency without evidence of thromboembolic complications or aneurysm formation. A foreign body immune response was observed with marked macrophage infiltration and giant cell formation by post-operative week 3. Organized vascular neotissue, consisting of endothelialization, medial generation, and collagen deposition, was evident within the internal lumen of the scaffolds by post-operative week 6. These results present the ability to create sub-1mm ID biodegradable tubular scaffolds that are functional as vascular grafts, and provide an experimental approach for the study of vascular tissue engineering using mouse models.
Figures





References
-
- Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, Langer R. Functional arteries grown in vivo. Science. 1999;284:489–493. - PubMed
-
- Watanabe M, Shin’oka T, Tohyama S, Hibino N, Konuma T, Matsumura G, Kosaka Y, Ishida T, Imai Y, Yamakawa M, Ikada Y, Morita S. Tissue engineered vascular autograft: inferior vena cava replacement in a dog model. Tissue Eng. 2001;7:429–439. - PubMed
-
- Campbell JH, Efendy JL, Campbell GR. Novel vascular graft grown within recipient’s own peritoneal cavity. Circ Res. 1999;85:1173–1178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

