Regional differences in the coupling of cerebral blood flow and oxygen metabolism changes in response to activation: implications for BOLD-fMRI
- PMID: 18164629
- PMCID: PMC2819080
- DOI: 10.1016/j.neuroimage.2007.11.015
Regional differences in the coupling of cerebral blood flow and oxygen metabolism changes in response to activation: implications for BOLD-fMRI
Abstract
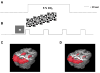
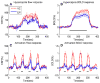
Functional magnetic resonance imaging (fMRI) based on blood oxygenation level dependent (BOLD) signal changes is a sensitive tool for mapping brain activation, but quantitative interpretation of the BOLD response is problematic. The BOLD response is primarily driven by cerebral blood flow (CBF) changes, but is moderated by M, a scaling parameter reflecting baseline deoxyhemoglobin, and n, the ratio of fractional changes in CBF to cerebral metabolic rate of oxygen consumption (CMRO(2)). We compared M and n between cortical (visual cortex, VC) and subcortical (lentiform nuclei, LN) regions using a quantitative approach based on calibrating the BOLD response with a hypercapnia experiment. Although M was similar in both regions (~5.8%), differences in n (2.21+/-0.03 in VC and 1.58+/-0.03 in LN; Cohen d=1.71) produced substantially weaker (~3.7x) subcortical than cortical BOLD responses relative to CMRO(2) changes. Because of this strong sensitivity to n, BOLD response amplitudes cannot be interpreted as a quantitative reflection of underlying metabolic changes, particularly when comparing cortical and subcortical regions.
Figures

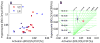



References
-
- Allison JD, Meador KJ, Loring DW, Figueroa RE, Wright JC. Functional MRI cerebral activation and deactivation during finger movement. Neurology. 2000;54:135–142. - PubMed
-
- Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. 2002;25:621–625. - PubMed
-
- Bandettini P. Functional MRI today. Int J Psychophysiol. 2007;63:138–145. - PubMed
-
- Behzadi Y, Liu TT. An arteriolar compliance model of the cerebral blood flow response to neural stimulus. NeuroImage. 2005;25:1100–1111. - PubMed
-
- Boxerman JL, Bandettini PA, Kwong KK, Baker JR, Davis TL, Rosen BR, Weisskoff RM. The intravascular contribution to fMRI signal change: Monte Carlo modeling and diffusion-weighted studies in vivo. Magn Reson Med. 1995a;34:4–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

